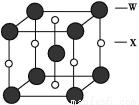
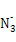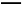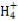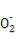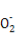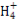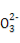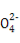ћвƒњƒЏ»Ё
ѕ¬Ѕ– µ—йѕ÷ѕуЋщґ‘”¶µƒјл„”Јљ≥ћ љ≤ї’э»Јµƒ «(°°°°)
µ—йѕ÷ѕујл„”Јљ≥ћ љ
A
H2SO4
KIµнЈџ»№“Ї‘Џњ’∆ш÷–Ј≈÷√“їґќ ±ЉдЇу»№“Ї≥ јґ…Ђ4H++4I-+O2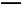 2I2+2H2O
2I2+2H2O
B
ѕ°—ќЋб
ѕ°ћЉЋбƒ∆»№“Їњ™ Љ ±ќё∆ш≈Ё,Їујі”–∆ш≈ЁC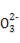 +H+
+H+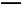 HC
HC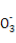
HC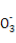 +H+
+H+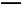 CO2°ь+H2O
CO2°ь+H2O
C
NaOH»№“Ї
¬»ЋЃ»№“Ї”…µ≠ї∆¬ћ…Ђ±дќ™ќё…ЂCl2+2OH-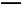 Cl-+ClO-+H2O
Cl-+ClO-+H2O
D
H2SO4Љ””–
Ј”ћ™µƒBa(OH)2
»№“Ї”–∞„…Ђ≥Ѕµн…ъ≥…,»№“Ї”…Їм…Ђ±дќ™ќё…ЂBa2++OH-+H++S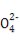
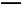 BaSO4°э+H2O
BaSO4°э+H2O
D
°Њљвќц°њI-Њя”–їє‘≠–‘,‘ЏЋб–‘ћхЉюѕ¬ƒ№±їњ’∆ш÷–µƒ—х∆ш—хїѓ,Aѕо’э»Ј;ѕ°HClѕтѕ°Na2CO3»№“Ї÷–µќЉ”,Јі”¶Ј÷Ѕљ≤љљш––:ҐўNa2CO3+HCl
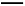 NaCl+NaHCO3,ҐЏNaHCO3+HCl
NaCl+NaHCO3,ҐЏNaHCO3+HCl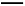 NaCl+H2O+CO2°ь,Bѕо’э»Ј;NaOH»№“Їѕт¬»ЋЃ÷–µќЉ”,Ѕљ’яЈі”¶:Cl2+2NaOH
NaCl+H2O+CO2°ь,Bѕо’э»Ј;NaOH»№“Їѕт¬»ЋЃ÷–µќЉ”,Ѕљ’яЈі”¶:Cl2+2NaOH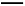 NaCl+NaClO+H2O,¬»ЋЃ—’…Ђ±д«≥÷Ѕѕы І,Cѕо’э»Ј;»№“Ї”…Їм…Ђ±дќ™ќё…Ђ,Ћµ√чBa(OH)2ѕыЇƒЌк,јл„”Јљ≥ћ љ”¶ќ™Ba2++2OH-+2H++S
NaCl+NaClO+H2O,¬»ЋЃ—’…Ђ±д«≥÷Ѕѕы І,Cѕо’э»Ј;»№“Ї”…Їм…Ђ±дќ™ќё…Ђ,Ћµ√чBa(OH)2ѕыЇƒЌк,јл„”Јљ≥ћ љ”¶ќ™Ba2++2OH-+2H++S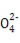
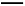 BaSO4°э+2H2O,Dѕоінќу°£
BaSO4°э+2H2O,Dѕоінќу°£

ЅЈѕ∞≤бѕµЅ–ір∞Є
ѕаєЎћвƒњ