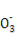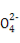МвДҝДЪИЭ
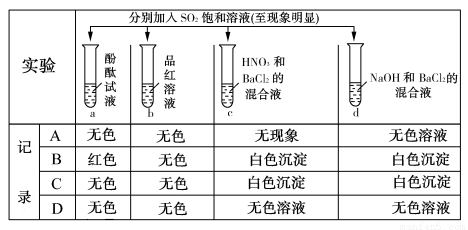
XЎўYЎўZЎўRЎўWКЗФӯЧУРтКэТАҙОФцҙуөДОеЦЦ¶МЦЬЖЪФӘЛШЎЈYәНRН¬ЦчЧе,ҝЙЧйіЙ№ІјЫ»ҜәПОпRY2,YәНZЧоНвІгөзЧУКэЦ®әНУлWөДЧоНвІгөзЧУКэПаН¬,25 ЎжКұ,0.1 mol/L XәНWРОіЙ»ҜәПОпөДЛ®ИЬТәpHОӘ1ЎЈПВБРЛө·ЁХэИ·өДКЗЈЁЎЎ ЎЎЈ©
A.УЙУЪПа¶ФФӯЧУЦКБҝR>Y,ЛщТФXЎўRЧйіЙ»ҜәПОпөД·РөгёЯУЪXЎўYЧйіЙөД»ҜәПОп
B.YәНЖдЛыЛДЦЦФӘЛШҫщҝЙРОіЙЦБЙЩБҪЦЦ¶юФӘ»ҜәПОп
C.RY2ДЬУлYЎўZРОіЙөДТ»ЦЦ»ҜәПОп·ҙУҰЙъіЙY2
D.YЎўZЎўWИэЦЦФӘЛШЧйіЙ»ҜәПОпөДЛ®ИЬТәТ»¶ЁПФјоРФ
B
ЎҫҪвОцЎҝУЙУЪ0.1 mol/L XәНWРОіЙ»ҜәПОпөДЛ®ИЬТәpH=1,ФтЛөГчXәНWРОіЙөД»ҜәПОпОӘЗҝЛб,№КXУҰОӘH,WУҰОӘClЎЈУЦТтYәНRН¬ЦчЧе,ҝЙЧйіЙ№ІјЫ»ҜәПОпRY2,ФтЦӘYОӘO,RОӘSЎЈУЦҫЭYәНZЧоНвІгөзЧУКэЦ®әНУлWЈЁClЈ©өДЧоНвІгөзЧУКэПаН¬,№КZОӘNaЎЈЛдПа¶ФФӯЧУЦКБҝS>O,ө«ТтH2O·ЦЧУјдҙжФЪЗвјь,өјЦВH2OөД·РөгёЯУЪH2S,AПоҙнОу;OУлHҝЙРОіЙH2OЎўH2O2,УлNaҝЙРОіЙNa2OЎўNa2O2,УлSҝЙРОіЙSO2ЎўSO3,УлClҝЙРОіЙCl2OЎўClO2ЎўCl2O7өИ,BПоХэИ·;SO2УлNa2O2·ҙУҰSO2+Na2O2 Na2SO4,ОЮO2ЙъіЙ,CПоҙнОу;YЎўZЎўWИэЦЦФӘЛШЧйіЙөД»ҜәПОпNaClO4өДЛ®ИЬТәіКЦРРФ,DПоҙнОуЎЈ
Na2SO4,ОЮO2ЙъіЙ,CПоҙнОу;YЎўZЎўWИэЦЦФӘЛШЧйіЙөД»ҜәПОпNaClO4өДЛ®ИЬТәіКЦРРФ,DПоҙнОуЎЈ

 И«ДЬІвҝШЖЪД©РЎЧҙФӘПөБРҙр°ё
И«ДЬІвҝШЖЪД©РЎЧҙФӘПөБРҙр°ё