��Ŀ����
0�棬1.01��105Paʱ���ֱ���ס��ҡ������������м���30.0mLͬŨ�ȵ����ᣬ
�ټ��벻ͬ�����������ֽ�����ɵĺϽ��ĩ�������ų��������������ڼס��ҡ������������м���������ͬ���ͬŨ�ȵ�����������Һ���ٷֱ����������ʵ����ͬ������ͬ�ֺϽ𣬲����ų��������������±���
���ݱ������ݿ��Լ���ó�����_________��
A.�Ͻ����� B.�Ͻ�����ֵĺ���
C.��������ʵ���Ũ�� D.������ܶ�
�ܼ����������ֵΪ__________________��
��2�������ֽ���������ѡ���е�ij���֣���Ӽ������е�����ʵ�飺
�ٲ������㣬�϶�û��________________������ĸ���ţ���
A.40Ca B.56Fe C.64Cu D.24Mg E.27Al
��ͨ�����������Ʋ�Ͻ�ijɷ�____________��
��3�����ݣ�2�����Ʋ���������x��y��ֵ�ֱ�Ϊ���٣�
| ��Ӧ���� | �� | �� | �� | |
| ����Ͻ�����(g) | 0.510 | 1.53 | 3.57 | |
| �����ᷴӦ��������(L) | 0.560 | 0.672 | 0.672 | |
| ���������Ʒ�Ӧ��������(L) | 0.336 | x | y | |
�ټ��벻ͬ�����������ֽ�����ɵĺϽ��ĩ�������ų��������������ڼס��ҡ������������м���������ͬ���ͬŨ�ȵ�����������Һ���ٷֱ����������ʵ����ͬ������ͬ�ֺϽ𣬲����ų��������������±���
���ݱ������ݿ��Լ���ó�����_________��
A.�Ͻ����� B.�Ͻ�����ֵĺ���
C.��������ʵ���Ũ�� D.������ܶ�
�ܼ����������ֵΪ__________________��
��2�������ֽ���������ѡ���е�ij���֣���Ӽ������е�����ʵ�飺
�ٲ������㣬�϶�û��________________������ĸ���ţ���
A.40Ca B.56Fe C.64Cu D.24Mg E.27Al
��ͨ�����������Ʋ�Ͻ�ijɷ�____________��
��3�����ݣ�2�����Ʋ���������x��y��ֵ�ֱ�Ϊ���٣�
��1��C 2mol/L ��2����C ��Mg��Al ��3��x=1.008L�� y=2.016L��
��1���ɱ������ݿ�֪�����������������������㣬���ɱ��������������������Ϊ0.06mol�����������������ʵ���Ũ��Ϊ2mol/L��
��2������֪�����кϽ������ᡢ����������Һ��Ӧ�ų�����������ȣ���˵���϶�û�������ᡢ����������Һ������Ӧ�Ľ�������ͭ�����ڣ�������ͭ������һ���������ᡢ�������Ʒ�Ӧ��������������Ҫô��ȣ�Ҫô��һ��Ϊ0��
�ںϽ��п϶���һ�ֽ���������������Һ��Ӧ������һ�ֽ���������������Һ����Ӧ����������������Һ��Ӧ�ų�������ֻ��ѡ���е�Ca��Al��
������Ca:
��n(Ca)=0.015mol��40g/mol=0.600g��0.510g�����ԺϽ��в���Ca�غ�Al����m(Al)=(0.015mol��2��27g/mol)/3=0.27g����һ�ֽ���������Ϊ0.51g-0.27g=0.24g�������������ᷴӦ����������Ϊ��0.560L-0.336L=0.224L����0.1mol���ʸý���ʧȥ1mol����ʱ���ĵ�����Ϊ12g����Mg��
��3�����Ͽ�֪��n(Mg):n(Al)=1:1��
������n(Mg)=n(Al)=0.03mol��
����n(Mg)=n(Al)=0.07mol��
��n(NaOH)=n(HCl)=0.06mol��������NaOH����������NaOH���㡣����Al~NaOH~1.5H2������x=0.03mol��1.5��22.4mol/L=1.008L��y=0.06mol��1.5��22.4mol/L=2.016L��
��2������֪�����кϽ������ᡢ����������Һ��Ӧ�ų�����������ȣ���˵���϶�û�������ᡢ����������Һ������Ӧ�Ľ�������ͭ�����ڣ�������ͭ������һ���������ᡢ�������Ʒ�Ӧ��������������Ҫô��ȣ�Ҫô��һ��Ϊ0��
�ںϽ��п϶���һ�ֽ���������������Һ��Ӧ������һ�ֽ���������������Һ����Ӧ����������������Һ��Ӧ�ų�������ֻ��ѡ���е�Ca��Al��
������Ca:
��n(Ca)=0.015mol��40g/mol=0.600g��0.510g�����ԺϽ��в���Ca�غ�Al����m(Al)=(0.015mol��2��27g/mol)/3=0.27g����һ�ֽ���������Ϊ0.51g-0.27g=0.24g�������������ᷴӦ����������Ϊ��0.560L-0.336L=0.224L����0.1mol���ʸý���ʧȥ1mol����ʱ���ĵ�����Ϊ12g����Mg��
��3�����Ͽ�֪��n(Mg):n(Al)=1:1��
������n(Mg)=n(Al)=0.03mol��
����n(Mg)=n(Al)=0.07mol��
��n(NaOH)=n(HCl)=0.06mol��������NaOH����������NaOH���㡣����Al~NaOH~1.5H2������x=0.03mol��1.5��22.4mol/L=1.008L��y=0.06mol��1.5��22.4mol/L=2.016L��

��ϰ��ϵ�д�
�����Ŀ
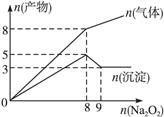
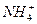 �Ļ��Һ�в��ȣ�������������������ʵ�����mol�������Na2O2�����ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����ԭ��Һ��Al3+��Mg2+��
�Ļ��Һ�в��ȣ�������������������ʵ�����mol�������Na2O2�����ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����ԭ��Һ��Al3+��Mg2+��