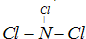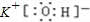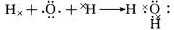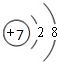��Ŀ����
����A��B��C��D��E���ֺ˵����С��18��Ԫ�أ����У�Aԭ�Ӻ��������ӣ�Bԭ�������������Ǵ�����2����Cԭ������������������Ӳ�����3����Dԭ�Ӻ���K���M���������1����E�γɵ�-1��������Arԭ�Ӻ�������Ų���ͬ��
�ɴ���֪��
��1��Aԭ�ӷ�����
��2��Bԭ�ӽṹʾ��ͼ��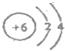
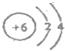
��3��EԪ���γɵĵ�����A��C��D��Ԫ���γɳʼ��ԵĻ����ﷴӦ�ķ���ʽ��
�ɴ���֪��
��1��Aԭ�ӷ�����
11H
11H
����2��Bԭ�ӽṹʾ��ͼ��
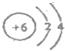
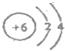
��3��EԪ���γɵĵ�����A��C��D��Ԫ���γɳʼ��ԵĻ����ﷴӦ�ķ���ʽ��
2NaOH+Cl2�TNaCl+NaClO+H2O
2NaOH+Cl2�TNaCl+NaClO+H2O
��������Aԭ�Ӻ��������ӣ�ӦΪHԪ�أ�
Bԭ�������������Ǵ�����2����ӦΪCԪ�أ�
Cԭ������������������Ӳ�����3����ӦΪOԪ�أ�
Dԭ�Ӻ���K���M���������1��ӦΪNaԪ�أ�
E�γɵ�-1��������Arԭ�Ӻ�������Ų���ͬ��ӦΪClԪ�أ�
Ȼ��������������
Bԭ�������������Ǵ�����2����ӦΪCԪ�أ�
Cԭ������������������Ӳ�����3����ӦΪOԪ�أ�
Dԭ�Ӻ���K���M���������1��ӦΪNaԪ�أ�
E�γɵ�-1��������Arԭ�Ӻ�������Ų���ͬ��ӦΪClԪ�أ�
Ȼ��������������
����⣺�������֪��AΪHԪ�أ�BΪCԪ�أ�CΪOԪ�أ�DΪNaԪ�أ�EΪClԪ�أ�
��1����AΪHԪ�أ���Aԭ�ӷ�����11H���ʴ�Ϊ��11H��
��2��BΪCԪ�أ�Cԭ�ӽṹʾ��ͼΪ��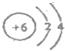 ���ʴ�Ϊ��
���ʴ�Ϊ��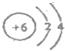 ��
��
��3��E�ĵ���ΪCl2��A��C��D��Ԫ���γɳʼ��ԵĻ�����ΪNaOH�����߷�Ӧ�ķ���ʽ��2NaOH+Cl2�TNaCl+NaClO+H2O���ʴ�Ϊ��2NaOH+Cl2�TNaCl+NaClO+H2O��
��1����AΪHԪ�أ���Aԭ�ӷ�����11H���ʴ�Ϊ��11H��
��2��BΪCԪ�أ�Cԭ�ӽṹʾ��ͼΪ��
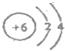 ���ʴ�Ϊ��
���ʴ�Ϊ��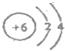 ��
����3��E�ĵ���ΪCl2��A��C��D��Ԫ���γɳʼ��ԵĻ�����ΪNaOH�����߷�Ӧ�ķ���ʽ��2NaOH+Cl2�TNaCl+NaClO+H2O���ʴ�Ϊ��2NaOH+Cl2�TNaCl+NaClO+H2O��
�����������ǶԺ�������Ų��Ŀ����⣬����Ĺؼ�������������ṩ����Ϣ����Ͽα�����ѧ��֪ʶ�����������ѧ������Ϣ��ʵ��Ӧ��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��