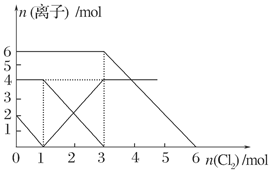
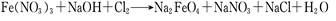��Ŀ����
��3.96g���ΪX2YZ4���Σ����Ǹ��Σ�����ˮ���μ�����ϡ������ټ���2.24g��ԭ���ۣ�ǡ����ȫ��Ӧ����Fe2+����Ӧ�����Һ�м���������KOH��Һ���պý�Fe2+��ȫ���������������ˡ�ϴ�Ӳ���ּ��Ⱥ�õ�����ɫ����4.80g������Һ��һ�������������ɵõ�һ�ִ����IJ����ᾧˮ�ĺ������Σ����Ǹ��Σ�13.92g��
��1������ɫ����Ļ�ѧʽΪ______________���������εĻ�ѧʽΪ______________��
��2�����ΪX2YZ4�������Ƿ�����Ԫ�غͼ�Ԫ�أ�ͨ������˵����
��3���ƶ�X2YZ4���εĻ�ѧʽ��д���ƶϹ��̡�
��1������ɫ����Ļ�ѧʽΪ______________���������εĻ�ѧʽΪ______________��
��2�����ΪX2YZ4�������Ƿ�����Ԫ�غͼ�Ԫ�أ�ͨ������˵����
��3���ƶ�X2YZ4���εĻ�ѧʽ��д���ƶϹ��̡�
��1����2�֣�Fe2O3��K2SO4��
��2����2�֣�������������ʵ���Ϊ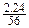 ��0.04(mol)����4.8gFe2O3�к��������ʵ���Ϊ
��0.04(mol)����4.8gFe2O3�к��������ʵ���Ϊ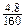 ��2��0.06(mol)��0.06>0.04,��X2YZ4�����к�����Ԫ�ء�
��2��0.06(mol)��0.06>0.04,��X2YZ4�����к�����Ԫ�ء�
13.92g K2SO4��K+�����ʵ���Ϊ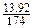 ��2��0.16(mol)��������Fe2+����KOH�����ʵ���Ϊ0.06��2��0.12(mol)��0.16>0.12����X2YZ4�����к��м�Ԫ�ء�
��2��0.16(mol)��������Fe2+����KOH�����ʵ���Ϊ0.06��2��0.12(mol)��0.16>0.12����X2YZ4�����к��м�Ԫ�ء�
��3����6�֣���֪X2YZ4���Σ��Һ�����Ԫ�غͼ�Ԫ�أ������бض���һ�ַǽ���Ԫ����Ϊ���ۡ�n(K) ��n(Fe)��0.04��0.02��2��1
����XΪFe��ZΪK������K��+1�ۣ���Fe�Ļ��ϼۡ�+2ʱ��Y�Ļ��ϼۡܣ�8����������
����XΪK��YΪFe����ZΪ�ǽ������ڻ�ѧʽ�гʸ��ۡ�
��X2YZ4��Ħ������Ϊ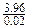 ��198(g/mol)����Z�����ԭ������Ϊ16����ZΪ��Ԫ�أ�������X2YZ4�Ļ�ѧʽΪK2FeO4��
��198(g/mol)����Z�����ԭ������Ϊ16����ZΪ��Ԫ�أ�������X2YZ4�Ļ�ѧʽΪK2FeO4��
��2����2�֣�������������ʵ���Ϊ
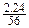 ��0.04(mol)����4.8gFe2O3�к��������ʵ���Ϊ
��0.04(mol)����4.8gFe2O3�к��������ʵ���Ϊ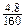 ��2��0.06(mol)��0.06>0.04,��X2YZ4�����к�����Ԫ�ء�
��2��0.06(mol)��0.06>0.04,��X2YZ4�����к�����Ԫ�ء�13.92g K2SO4��K+�����ʵ���Ϊ
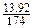 ��2��0.16(mol)��������Fe2+����KOH�����ʵ���Ϊ0.06��2��0.12(mol)��0.16>0.12����X2YZ4�����к��м�Ԫ�ء�
��2��0.16(mol)��������Fe2+����KOH�����ʵ���Ϊ0.06��2��0.12(mol)��0.16>0.12����X2YZ4�����к��м�Ԫ�ء���3����6�֣���֪X2YZ4���Σ��Һ�����Ԫ�غͼ�Ԫ�أ������бض���һ�ַǽ���Ԫ����Ϊ���ۡ�n(K) ��n(Fe)��0.04��0.02��2��1
����XΪFe��ZΪK������K��+1�ۣ���Fe�Ļ��ϼۡ�+2ʱ��Y�Ļ��ϼۡܣ�8����������
����XΪK��YΪFe����ZΪ�ǽ������ڻ�ѧʽ�гʸ��ۡ�
��X2YZ4��Ħ������Ϊ
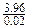 ��198(g/mol)����Z�����ԭ������Ϊ16����ZΪ��Ԫ�أ�������X2YZ4�Ļ�ѧʽΪK2FeO4��
��198(g/mol)����Z�����ԭ������Ϊ16����ZΪ��Ԫ�أ�������X2YZ4�Ļ�ѧʽΪK2FeO4����

��ϰ��ϵ�д�
 һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�
�����Ŀ