��Ŀ����
�Ų��������Ļ�ѧ��������������Fe2O3�������ǵ��ӡ����Ź�ҵ�Ĵ��Բ��ϣ���ҵ�ϲ��������Ѱ۵��½��ϣ�������FeSO4��Һ���Ʊ��Ų��������IJ������£�
�����½��ϣ�������FeSO4��Һ���м�������2 mo1��L-1��H2SO4����Ƥ��
�������������Һ�м���������ˮ�������˳�ȥ���������ʵ���������
�۽������õ���ҺŨ���ᾧ�õ�����A��
�ܽ�����A����ˮ��������NH4HCO3������CO2����ͬʱ�õ�FeCO3��������ɫ��ҺC��
�ݽ�FeCO3����ϴ�ӡ���ɲ����ա��������еı仯Ϊ��FeCO3="FeO+CO2��; " 4FeO+O2="2" Fe2O3��
��������Ϣ�ش��������⣺
��1����18.4mo1��L-1��H2SO4����500mL 2 mo1��L-1H2SO4�����貣��������
mL��Ͳ�����������ձ���500mI������ƿ�⣬����Ҫ ��
��2���������2 mo1��L-1H2SO4����Ƥ�����÷ֱ�Ϊ ��
��3������A�Ļ�ѧʽΪ ��������ҺC�����������ӵķ����� ��
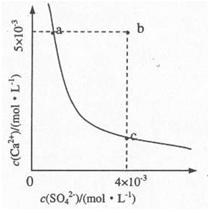
��4������ҺC�м���CaCl2��Һ�ܵõ�CaSO4������������KSP��CaSO4��=9x10-6��������CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ��
��a���Ӧ��KSP c���Ӧ��KSP����ڡ�����С�ڡ����ڡ�����
������b��䵽a�����д�ʩ���е��� ��

�����½��ϣ�������FeSO4��Һ���м�������2 mo1��L-1��H2SO4����Ƥ��
�������������Һ�м���������ˮ�������˳�ȥ���������ʵ���������
�۽������õ���ҺŨ���ᾧ�õ�����A��
�ܽ�����A����ˮ��������NH4HCO3������CO2����ͬʱ�õ�FeCO3��������ɫ��ҺC��
�ݽ�FeCO3����ϴ�ӡ���ɲ����ա��������еı仯Ϊ��FeCO3="FeO+CO2��; " 4FeO+O2="2" Fe2O3��
��������Ϣ�ش��������⣺
��1����18.4mo1��L-1��H2SO4����500mL 2 mo1��L-1H2SO4�����貣��������
mL��Ͳ�����������ձ���500mI������ƿ�⣬����Ҫ ��
��2���������2 mo1��L-1H2SO4����Ƥ�����÷ֱ�Ϊ ��
��3������A�Ļ�ѧʽΪ ��������ҺC�����������ӵķ����� ��
��4������ҺC�м���CaCl2��Һ�ܵõ�CaSO4������������KSP��CaSO4��=9x10-6��������CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ��
��a���Ӧ��KSP c���Ӧ��KSP����ڡ�����С�ڡ����ڡ�����
������b��䵽a�����д�ʩ���е��� ��
| A����������CaCl2 | B����������BaCl2�� |
| C����������Na2SO4 | D������ |
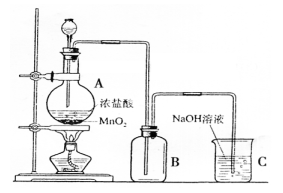
��1��100����2�֣���ͷ�ιܣ�1�֣� ��2����2mol��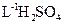 ��ֹFe
��ֹFe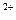 ����ˮ�⣬��1�֣� ����Ƥ��ֹFe
����ˮ�⣬��1�֣� ����Ƥ��ֹFe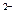 ��������1�֣� ��3��
��������1�֣� ��3��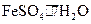 ����1�֣�ȡ����Һ������������������Һ�����ȣ���������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤������Һ��������Ϊ
����1�֣�ȡ����Һ������������������Һ�����ȣ���������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤������Һ��������Ϊ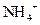 ����3�֣� ��4�����ڣ���2�֣� A��B����2�֣�
����3�֣� ��4�����ڣ���2�֣� A��B����2�֣�
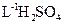 ��ֹFe
��ֹFe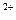 ����ˮ�⣬��1�֣� ����Ƥ��ֹFe
����ˮ�⣬��1�֣� ����Ƥ��ֹFe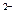 ��������1�֣� ��3��
��������1�֣� ��3��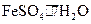 ����1�֣�ȡ����Һ������������������Һ�����ȣ���������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤������Һ��������Ϊ
����1�֣�ȡ����Һ������������������Һ�����ȣ���������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤������Һ��������Ϊ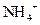 ����3�֣� ��4�����ڣ���2�֣� A��B����2�֣�
����3�֣� ��4�����ڣ���2�֣� A��B����2�֣���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ