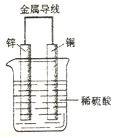
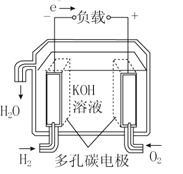
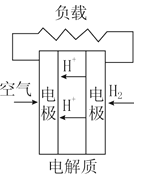
��Ŀ����
����Ŀ�����ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ����±���
Ԫ�ش��� | X | Y | Z | M | R | Q | U | |
ԭ�Ӱ뾶�� | 1.86 | 0.99 | 1.43 | 1.60 | 0.75 | 0.74 | 0.82 | |
��Ҫ���ϼ� | ������� | +1 | +7 | +3 | +2 | +5 | +3 | |
����� | -1 | -3 | -2 | |||||
����˵��������ǣ� ��
A. Ԫ��X��Q�γɵĻ������в����ܺ��й��ۼ�
B. X��Z��R������������ˮ����֮����������Ӧ
C. R3-��Q2-������ʧȥ����
D. M(OH)2�ļ��Ա�XOH�ļ�����
���𰸡�A
��������
ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ����������������������Ͻ�Ԫ��ԭ�Ӱ뾶��С�����½�Ԫ��ԭ�Ӱ뾶���Ԫ��������۵�������������ͬһ�ǽ���Ԫ�����������������۵ľ���ֵ֮��Ϊ8��Xԭ�Ӱ뾶������������Ϊ+1����XΪNaԪ�أ�YԪ�����������+7���������-1�ۣ���YΪClԪ�أ�Z��UԪ���������Ϊ+3��Ϊ��IIIA��Ԫ�أ���Zԭ�Ӱ뾶����ClԪ�أ�����Z��AlԪ�أ�U��BԪ�أ�MԪ���������Ϊ+2��Ϊ��IIA��Ԫ�أ���ԭ�Ӱ뾶����Z������M��MgԪ�أ�RԪ���������Ϊ+5�������Ϊ-3������RΪ��VA��Ԫ�أ���ԭ�Ӱ뾶С��ClԪ�أ�ΪNԪ�أ�QԪ�������Ϊ-2��û��������ۣ�ΪOԪ�أ��ݴ˽��
�������Ϸ�����֪X��Y��Z��M��R��Q��U�ֱ���Na��Cl��Al��Mg��N��O��B��
A��Ԫ��X��Q�γɵĻ�������Na2O��Na2O2�����������к��й��ۼ���A����
B��X��Z��R������������ˮ����ֱ���NaOH��Al��OH��3��HNO3�����������������ԣ���������֮����������Ӧ��B��ȷ��
C���ǽ�����Խ����Ԫ�أ��������ӻ�ԭ��Խǿ���ǽ�����O��NԪ�أ���R3-��Q2-������ʧȥ���ӣ�C��ȷ��
D��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ��M����������X������Mg��OH��2�ļ��Ա�NaOH�ļ�������D��ȷ��
��ѡA��

����Ŀ��ijѧ����0.2000mol��L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��������£�
�ٹ̶��õζ���F��ʹ�ζ��ܼ������Һ�壻
��������ˮϴ�Ӽ�ʽ�ζ���F��������ע��NaOH��Һ����0���̶������ϣ�
���ñ�Һ�ζ����յ㣬���µζ���Һ�������
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ��
������Һ������0������0���̶������£������¶�����
��ش�
��1�����ϲ��������˳��Ϊ�����ţ�____________________��
��2����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����е�________��Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��
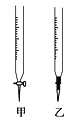

��3���ζ���F��Ӧ��ѡ����ͼ�еζ���_____�����ţ���
��4����������ᵼ�²ⶨ���������ƫ��������ƫС��������Ӱ������
A��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ___________��
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�����������ȷ___________��
C��������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ___________��
D�����Ʊ���Һ��NaOH�л���KOH����___________��
��5���ζ�������,����_____________,����______________,�۾�Ӧ�۲죨���ţ�_________A.�ζ�����Һ��ı仯 B.��ƿ����Һ��ɫ�ı仯
��6���жϵζ��յ�������ǣ���ƿ����Һ______________________________��
��7������ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ________mL��
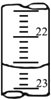
��8�������������ݣ���������������Һ��Ũ�ȣ�______mol/L��
�ζ����� | ���������mL�� | ���ռ������mL�� | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 20.00 | 0.40 | 20.40 |
�ڶ��� | 20.00 | 2.00 | 24.10 |
������ | 20.00 | 4.00 | 24.00 |
����Ŀ����ѧ��Ӧ���������仯�����Ȼ�����ǻ�ѧ��Ӧ�������仯����Ҫ��ʽ֮һ��
���飨C3H8����һ��������ȼ�ϣ���ͼ��һ����������ȫȼ������CO2��1molH2O��I�������е������仯ͼ���Իش��������⣺
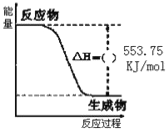
��1��д������ȼ�յ��Ȼ�ѧ����ʽ��__________��
��2�������ѣ�CH3OCH3����һ������ȼ�ϣ�Ӧ��ǰ��������1mol��������ȫȼ������CO2��Һ̬ˮ�ų�1455kJ����������1mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1645kJ�����������������б���Ͷ����ѵ����ʵ���֮��Ϊ__________��
������ͼ��ʾ��װ�ý����к��ȵIJⶨʵ�飬�ֱ�ȡ50mL0.55mol/L��NaOH��Һ��50mL0.25mol/L���������ʵ�飬�ش��������⣺

��1������ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��__________������֮�⣬װ���е�һ�����Դ�����__________��
��2��������Ϊ0.55mol/L��NaOH��Һ��0.25mol/L��������Һ���ܶȶ���1g/cm3���кͺ�������Һ�ı�����c=4.18J/(g����)��ͨ���������ݼ����к��ȡ�H=__________���������С�����һλ����
�¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | ||
H2SO4 | NaOH | ƽ��ֵ | ||
1 | 26.2 | 26.0 | 26.1 | 29.5 |
2 | 27.0 | 27.4 | 27.2 | 32.3 |
3 | 25.9 | 25.9 | 25.9 | 29.2 |
4 | 26.4 | 26.2 | 26.3 | 29.8 |
��3��������60mL0.25mol/L��H2SO4��50mL0.55mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������__________�����ȡ�����ȡ�����
��4������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ��_____��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d����������ʵ������¶Ⱦ��������ƽ��ֵ