��Ŀ����
����Ŀ����ͼ��ʾ��ʾһЩ�����е�ijЩ�ṹ�����Ƿֱ���NaCl��CsCl���ɱ������ʯ��ʯī����ṹ�е�ijһ�ֵ�ijһ���֡�
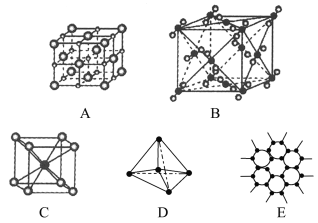
(1)���д������ʯ����(������ĸ����ͬ)________������ÿ��̼ԭ����________��̼ԭ����ӽ��Ҿ�����ȡ����ʯ����________���塣
(2)���д���ʯī����________������ÿ����������ռ��̼ԭ����ƽ��Ϊ________����
(3)���д���NaCl�������________��ÿ��Na����Χ������ӽ��Ҿ�����ȵ�Na����________����
(4)����CsCl�������________��������________���壬ÿ��Cs����________��Cl�����ڡ�
(5)�����ɱ�����________��������________���壬ÿ��CO2������________��CO2���ӽ��ڡ�
(6)�������������۵��ɸߵ��͵�����˳��Ϊ________��
���𰸡�D 4 ԭ�� E 2 A 12 C ���� 8 B ���� 12 ʯī![]() ���ʯ
���ʯ![]() �ɱ�
�ɱ�
��������
A�У��뾶�ϴ��ԭ�Ӹ���=![]() =4���뾶��С��ԭ�Ӹ���=
=4���뾶��С��ԭ�Ӹ���=![]() =4��
=4��
B�У��þ����ɷ��ӹ��ɣ�
C�У���ɫС����������ԭ�ӵĸ���=1����ɫС����������ԭ�ӵĸ���=![]() ��
��
D�У�ֻ��һ��ԭ�ӣ���ÿ��ԭ����4��ԭ��ͨ�����ۼ��γ�������ṹ��
E�У�ֻ��һ��ԭ�ӣ���ԭ��֮��ͨ�����ۼ��γɲ�״�ṹ���ݴ˽��
(1)���ʯ�ṹ�е�ÿ��Cԭ�������ڵ�4��Cԭ���γ��������壬����ͼDΪ���ʯ��ÿ��̼ԭ����4��̼ԭ������Ҿ�����ȡ����ʯ�ǿռ���״�ṹ������ԭ�Ӿ��壬�ʴ�Ϊ��D��4��ԭ�ӣ�
(2)ʯī�Dz�״�ṹ���ڲ����֮���Է��»�������ã��ڲ���̼��̼�Թ��ۼ�����ã��γ������Σ�����ͼEΪʯī�Ľṹ��ÿ����������ռ�е�̼ԭ����ƽ��Ϊ6��![]() =
=![]() ���ʴ�Ϊ��E��2��
���ʴ�Ϊ��E��2��
(3)��NaCl�����У�ÿ����������Χ�����������ӣ�ÿ����������ΧҲ�����������ӣ�����ͼAΪNaCl�Ľṹ�����ݾ����Ľṹ��ÿ����������Χ�����������������С���������Խ��ߵ�λ�ã�ÿ����������Χ�а˸������������壬����ÿ����������Χ��������Ҿ�����ȵ������Ӿ���12�����ʴ�Ϊ��A��12��
(4)CsCl�ľ���������Ӻ������ӵ���λ������8����ÿ���������Χ��8�������ӣ�ÿ����������ΧҲ��8������ӣ�����ͼCΪCsCl�Ľṹ���������Ӿ��壬�ʴ�Ϊ��C�����ӣ�8��
(5)�ɱ��Ƿ��Ӿ��壬![]() ����λ��������Ķ���������ϣ��Զ����ϵ�
����λ��������Ķ���������ϣ��Զ����ϵ�![]() ����Ϊ�����������������
����Ϊ�����������������![]() ���ӷֲ�����ö���������12����������ϣ�����ͼBΪ�ɱ����壬�ʴ�Ϊ��B�����ӣ�12��
���ӷֲ�����ö���������12����������ϣ�����ͼBΪ�ɱ����壬�ʴ�Ϊ��B�����ӣ�12��
(6)һ����˵�����־�����۵��ϵΪ��ԭ�Ӿ���![]() ���Ӿ���
���Ӿ���![]() ���Ӿ��壬ʯī���۵���ڽ��ʯ�������Ӿ�����뾶Խ������ԽС���۵�Խ�ͣ���������Ӱ뾶���������ӣ�ʯī���۵���ڽ��ʯ�������۵��ɸߵ��͵�����˳��Ϊ��ʯī
���Ӿ��壬ʯī���۵���ڽ��ʯ�������Ӿ�����뾶Խ������ԽС���۵�Խ�ͣ���������Ӱ뾶���������ӣ�ʯī���۵���ڽ��ʯ�������۵��ɸߵ��͵�����˳��Ϊ��ʯī![]() ���ʯ
���ʯ![]() �ɱ����ʴ�Ϊ��ʯī
�ɱ����ʴ�Ϊ��ʯī![]() ���ʯ
���ʯ![]() �ɱ���
�ɱ���

����Ŀ��A��B��C��D��E��F��G��ǰ������Ԫ�أ�ԭ���������������ݱ����ṩ���й���Ϣ���ش����⣺
Ԫ�� | �����Ϣ |
A | ���е������ܶ���С |
B | �γɻ�������������Ԫ�� |
D | ��̬ԭ����ֻ��3���ܼ�����2��δ�ɶԵ��� |
E | ��������ԭ�Ӱ뾶��� |
F | ���������е縺������Ԫ�� |
G | �����ֻ��һ�����ӣ��ڲ��������� |
��1��EԪ�������ڱ��е�λ����______��FԪ��ԭ�����������Ų�ʽΪ______��BD2�����幹��Ϊ______��
��2����D��E��F����Ԫ���γɵ�һ�ֳ������ʵ�ˮ��Һ�Լ��ԣ������ӷ���ʽ��ʾ���Լ��Ե�ԭ��______��
��3���⻯���ȶ��ԣ�B______D������������Ӧ��ˮ�������ԣ�C______F��(����������������)
��4��ÿ��B2A4�����к���______��������______���м���
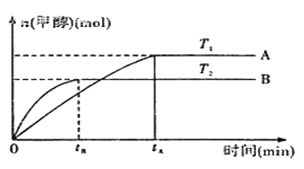
����Ŀ��̼��������ȡ�������̻������ԭ�ϣ�Ҳ����������Ĵ����ȡ�һ�ֱ����Ȼ�狀����̿���Ʊ��ߴ���̼���̵Ĺ���������ͼ��ʾ
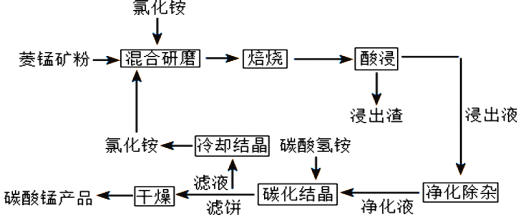
��֪�����̿�۵���Ҫ�ɷ���MnCO3������������Fe��Al��Ca��Mg��Ԫ��
�ڳ����£���ؽ���������Ũ��Ϊ0.1mol/Lʱ�γ�M(OH)n������pH��Χ���
�������� | Al3+ | Fe3+ | Fe2+ | Ca2+ | Mn2+ | Mg2+ |
��ʼ������pH | 3.8 | 1.5 | 6.3 | 10.6 | 8.8 | 9.6 |
������ȫ��pH | 5.2 | 2.8 | 8.3 | 12.6 | 10.8 | 11.6 |
�ش��������⣺
(1)�������ĥ��������Ϊ_______________________
(2)��������ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ_________________________________
(3)����ͼ1��ͼ2�������Ȼ�李����̿�۵����������_____________________________
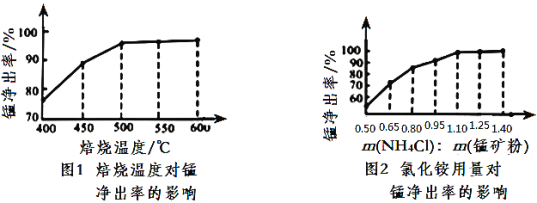
(4)����������������
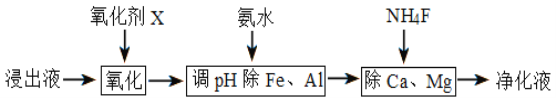
����֪������������������ǿ��˳��Ϊ(NH4)2S2O8��KMnO4��MnO2��Fe3+����������X��ѡ��__________
A��(NH4)2S2O8 B��MnO2 C��KMnO4
�ڵ���pHʱ��pH��ȡ�ķ�ΧΪ_________________
(5)��̼���ᾧ�������в�����̼��林���̼����泥����ܵ�ԭ����__________________