��Ŀ����
����Ŀ�����仯�������ִ������������з�����Ҫ���á�
(1)����ͨ���Ȼ�ѧѭ���ڽϵ��¶���������ֽ��Ʊ�������
SO2(g)+I2(s)+2H2O(1)==2HI(aq)+H2SO4(aq) ��H1=��151.5kJ��mol-1
2HI(aq)==H2(g)+I2(s) ��H2=+110kJ��mol-1
H2S(g)+H2SO4(aq)==S(s)+SO2(g)+2H2O(I) ��H3=+65kJ��mol-1
�Ȼ�ѧ���ѭ������ֽ�������������ǵ��Ȼ�ѧ����ʽΪ_________________��
(2)���᳧β���к��д���SO2��Ϊ����������ʿ���ͼ1��ʾװ��(�缫��Ϊ���Ե缫)�������գ������������(Li��SO2C12)��ؿ���Ϊ��Դ����Ʊ�Ni(H2PO2)2(ͼ2)����֪��ط�ӦΪ��2Li+SO2C12=2LiCl+SO2��
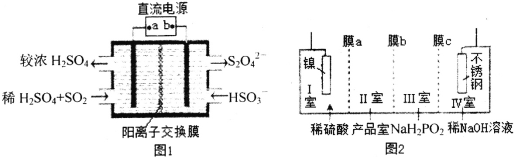
��ͼ1�У�aΪֱ����Դ��______��(����������������)���������ĵ缫��ӦʽΪ_____��
��SO2C12������Sԭ�ӵ��ӻ���ʽΪ____________________��
����������ȵ�ص�������ӦʽΪ_______________________��
��ͼ2��ĤaΪ______����Ĥ(��������������������������ͬ)��ĤcΪ_________����Ĥ������ֵ缫�ĵ缫��ӦʽΪ_______________________________��
(3)��������Ĵ������ǹ�ҵ�������������Ҫ��ӦO2(g)+2SO2(g) ![]() 2SO3(g)��
2SO3(g)��
��֪����ƽ�ⳣ��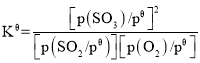 ������
������![]() Ϊ��ѹǿ(1��105Pa)��p(SO3)��p(O2)��p(SO2)Ϊ����ֵ�ƽ���ѹ����p(SO3)=x(SO3)p��pΪƽ����ѹ��x(SO3)Ϊƽ��ϵͳ��SO3�����ʵ���������SO2��O2��ʼ���ʵ���֮��Ϊ2��1����Ӧ�ں㶨�¶Ⱥͱ�ѹǿ�½��У�SO3��ƽ�����Ϊ
Ϊ��ѹǿ(1��105Pa)��p(SO3)��p(O2)��p(SO2)Ϊ����ֵ�ƽ���ѹ����p(SO3)=x(SO3)p��pΪƽ����ѹ��x(SO3)Ϊƽ��ϵͳ��SO3�����ʵ���������SO2��O2��ʼ���ʵ���֮��Ϊ2��1����Ӧ�ں㶨�¶Ⱥͱ�ѹǿ�½��У�SO3��ƽ�����Ϊ![]() ����
����![]() _______(�ú�
_______(�ú�![]() �����ʽ��ʾ)��
�����ʽ��ʾ)��
���𰸡�H2S(g) =H2(g)��S(s) ��H=+23.5 kJ ��mol��1 �� 2HSO3����2e����2H+= S2O42����2H2O sp3 SO2Cl2��2e��=2Cl����SO2�� ������ ������ 2H2O��2e��=H2����2OH�� ![]()
��������
��1��������֪�ʱ���Ȼ�ѧ����ʽ�Ƶ�����Ӧ���ٸ��ݸ�˹��������H��
��2���ٸ���ͼ1�ұߵ�SԪ�ػ��ϼ۱仯�жϼ��ԣ���ԭ������������ʱ����Դ������������������������жϵ缫a���ԣ���������ΪHSO3���ŵ�����S2O42�������ݵ缫��Ӧʽ��д������д�缫��Ӧʽ��
�ڸ���SO2Cl2��Sԭ�ӵĵ����Ų�����ϵ���ʽ��֪��Sԭ��������¶Ե��ӣ�����֪Sԭ�ӵ��ӻ���ʽ��
����д���-�����ȵ�صĸ�����Ӧʽ�������ܷ�Ӧ��ȥ������Ӧʽ���ɵó�������Ӧʽ��
�ܽ�Ͻ���Ĥ�����ã�ֻ����ij������ͨ����Ŀ��Ϊ�ٽ�ij�ֲ�������ɣ����ڵ��Ŀ��Ϊ�Ʊ�Ni��H2PO2��2����������缫��ŵ��ܽ�����������������������Ҫ��������γɲ�Ʒ������֪Ĥa�����ͣ����������������ҪH���ŵ磬������е�H����������ң�����ֵ缫Ϊˮ�е�H���ŵ����H2����ϵ缫��Ӧʽ��д���ɿɵò���ֵ缫�ĵ缫��Ӧʽ��
��3������SO2��O2��ʼ���ʵ���֮��Ϊ2��1����Ӧ�ں㶨�¶Ⱥͱ�ѹǿ�½��У�SO3��ƽ�����Ϊ������ɵó�SO3�ı仯��Ϊ2����������ʽ���ֱ����ƽ��ʱ�����ʵķ�ѹ�������ƽ�ⳣ��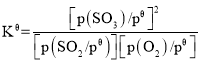 �м��ɵĽ����
�м��ɵĽ����
��1����Ӧ��+��Ӧ�ٿ���ȥH2SO4������ټ��Ϸ�Ӧ����ȥI2���ɵó�����ֽ�������������ǵ��Ȼ�ѧ����ʽ��H2S��g���TH2��g��+S��s����H����ϸ�˹���ɣ���H=��H3����H1����H2=65kJ��mol��1+110kJ��mol��1-151.5kJ��mol��1=+23.5kJ��mol��1���ʴ�Ϊ��H2S��g���TH2��g��+S��s����H=+23.5kJ��mol��1��
��2������ͼ1��֪���ұߵ缫��ӦΪHSO3����Ӧ����S2O42����SԪ�ػ��ϼ۽��ͣ������ұ�Ϊ���ص������������Ϊ���ص�����������Ϊ�������Դ��������������aΪֱ����Դ����������ϵ缫����ʽ��дԭ����ת���غ㡢����غ㣻�����ĵ缫��ӦʽΪ2HSO3��+2e��+2H��=S2O42��+2H2O���ʴ�Ϊ������2HSO3��+2e��+2H��=S2O42��+2H2O��
��SO2Cl2������Sԭ���¶Ե��ӣ�����Sԭ�ӵ��ӻ���ʽΪsp3���ʴ�Ϊ��sp3��
���-�����ȵ�صĸ���ΪLi-e��=Li������֪��ط�ӦΪ��2Li+SO2Cl2�T2LiCl+SO2�������ܷ�Ӧ-������Ӧ���ɵó�������ӦΪ��SO2Cl2+2e���T2Cl��+SO2�����ʴ�Ϊ��SO2Cl2+2e���T2Cl��+SO2����
�ܸ��ݵ��Ŀ�ģ��Ʊ�Ni��H2PO2��2����֪�����е����缫�������ŵ��ܽ��γ�Ni2����Ni2����Ҫ�����ƶ������Ʒ���γɲ�Ʒ������ĤaΪ�����ӽ���Ĥ�������Ϊ������������Ҫ��Һ�е������ӷŵ磬������е�H����Ҫͨ�������ӽ���Ĥ������ң�����ĤcΪ�����ӽ���Ĥ������ֵĵ缫��ӦΪH2O�е�H���ŵ����H2���缫��ӦʽΪ��2H2O+2e���TH2��+2OH�����ʴ�Ϊ�������ӣ������ӣ�2H2O+2e���TH2��+2OH����
��3����֪����ƽ�ⳣ��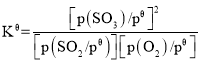 ��������K��=
��������K��=![]() SO2��O2��ʼ���ʵ���֮��Ϊ2��1��SO3��ƽ�����Ϊ������SO/span>3�ı仯��Ϊ2����������ʽ���£�
SO2��O2��ʼ���ʵ���֮��Ϊ2��1��SO3��ƽ�����Ϊ������SO/span>3�ı仯��Ϊ2����������ʽ���£�
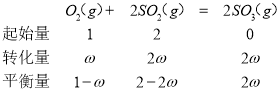
��p��O2��=![]() ,
,
p��SO2��=![]() ,
,
p��SO3��=![]() ,
,
����Ϊp=p������K��=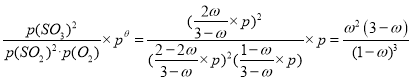 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�����Ŀ�������Dzݱ�ֲ�ﳣ���еijɷ֡�����������ϵõ����ᾧ��(H2C2O4��3H2O)�����ε����������
�۷е� | ��ɫ���ܽ��� | ���ֻ�ѧ���� | �� |
�۵㣺101-102�� �е㣺150-160������ | ���ᾧ����ɫ��������ˮ | 100.1��������ˮ��175�����Ϸֽ�����壻���л�ԭ�� | �������������ˮ |
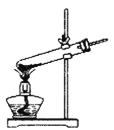
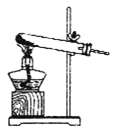
��1�����ᾧ��(H2C2O4��3H2O)175�����ϻᷢ���ֽ��������������ijʵ��С����ͨ��ʵ��֤�������������
�ٸ�С��ѡ��װ�ñ���Ϊ�ֽ�װ�ã���ѡ�ü�װ�õ�ԭ����_______����װ���������װ�õ��ŵ���________��ʵ��ǰ�����װ�������ԵIJ���������________��
�� ��
�� ��
��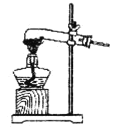
�ڴ�ͼ��ѡ�ú��ʵ�װ�ã���֤�ֽ���������壬װ�õ�����˳����______________��(��װ�ñ�ű�ʾ��ijЩװ�ÿ����ظ�ʹ�ã�Ҳ����װ��ͬ���Լ�)
B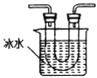 C
C D
D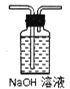 E
E![]() F
F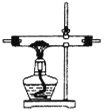
��Bװ�õ�������__________��
��2��ijʵ��С���ȡ4.0g�ֲ��ᾧ�����100mL��Һ������0.1mol��L-1���Ը��������Һ�ζ��ò�����Һ���ⶨ�ò��ᾧ��Ĵ��ȡ�
�����Ʋ�����Һ��Ҫ�õ�����Ҫ����������_______________��
�ڱ�ʵ��ﵽ�ζ��յ�ı�־��___________��
�۽���������Ϊ�ĵȷݣ�ʵ����ÿ��ƽ���������Ը��������Һ20mL������ôֲ����к����ᾧ�������Ϊ_______g(������λ��Ч����)��