��Ŀ����
ij�����ĵ�������к���ͭ�����Ƚ��������Ϊʵ����Դ�Ļ������ò���Ч��ֹ������Ⱦ��������¹������̣�
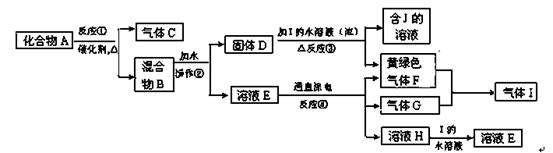
��1����������H2O2��Ŀ���� ����pH�����м�����Լ������ ���ѧʽ����ʵ���ҽ��й��˲������õ��IJ��������� ��
��2�����CuSO4��Һ��ԭ���� ����CuSO4��Һ�м���һ������NaCl��Na2SO3���������ɰ�ɫ��CuCl������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��CuCl��Ʒ��CuCl��������������96��50%Ϊ���Һϸ������ȡ���Ʊ���CuCl��Ʒ0��2500g����һ������0��5mol��L-1FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20mL����0.1000mol��L-1��Ce��SO4��2��Һ�ζ��������յ�ʱ����Ce��SO4��2��Һ24��60mL���йصĻ�ѧ��ӦΪ��
Fe3����CuCl��Fe2����Cu2����Cl����Ce4����Fe2����Fe3����Ce3����ͨ������˵����CuCl��Ʒ ������ϡ������ϡ������ұ���
��4��25��ʱ��KSP [Fe��OH��3]= 4.0��10-38��Fe3+����ˮ�ⷴӦFe3����3H2O Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ��Ϊ ��
Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ��Ϊ ��
��14�֣���1����Fe2+������Fe3�������ڵ���pHֵ��Cu2+���루2�֣���
CuO��Cu(OH)2��CuCO3�ȣ�2�֣���©�����ձ�����������2�֣�
��2��������Һ�е�H2O2������Ӱ����һ��CuCl�����ɣ�2�֣�
2CuSO4��2NaCl��Na2SO3��H2O��2CuCl����2Na2SO4��H2SO4��2�֣�
��3�����ϣ�2�֣� ��4��2.5��10��5��2�֣�
���������������1����������к���ͭ�����Ƚ���������������Ҫ���ܽ�ͭ�����Ƚ��������˫��ˮ��ǿ�����ԣ���������ԭ�Ե����ʣ�Fe2+���л�ԭ�ԣ���������H2O2��Fe2+�ܱ�˫��ˮ����ΪFe3�������ڵ���pHֵ��Cu2+���룻�ڵ���pH�����м�����Լ���������ȥ��Һ�е��ᣬ�Ҳ��������µ����ʣ�����Ҫ������Լ�������CuO��Cu(OH)2��CuCO3�����ݱ������ݿ�֪������Һ��pHֵ4.4ʱ��ͭ���ӿ�ʼ���ֳ���������Һ��pHֵΪ3.2ʱ�����������ӳ�����ȫ��ͭ����δ��������������Ҫʹ���������Ӻ�ͭ���ӷ��룬��Һ��pHӦ����3.2��pH��4.4.���ɵ�����������������ͨ������ʵ�ַ��룬���˲��õ�������������̨��©�����ձ����������ȣ��������ڲ���������©�����ձ�����������
��2����Ӧ�й��������ǹ����ģ�����������������ԣ��������ȥ��Ӱ��CuCl�����ɡ�˫��ˮ�����ֽ⣬�������CuSO4��Һ��ԭ���dz�����Һ�е�H2O2������Ӱ����һ��CuCl�����ɣ���ΪCuSO4�У�2�۵�ͭ�ܰ�Na2SO3�У�4�۵��������ɣ�6�۵�����2�۵�ͭ����ԭ���ɣ�1�۵�ͭ����������CuCl���������Ը÷�Ӧ�Ļ�ѧ����ʽΪ2CuSO4��2NaCl��Na2SO3��H2O��2CuCl����2Na2SO4��H2SO4��
��3������Ʒ��CuCl������Ϊx���йصĻ�ѧ��ӦΪFe3����CuCl��Fe2����Cu2����Cl����Ce4����Fe2����Fe3����Ce3�������ɻ�ѧ��Ӧ����ʽ��֪��CuCl������Fe2+������Ce4+
1 1
n(CuCl) 24.60��10-3L��0.1000 mol/L
��� n(CuCl)��24.60��10-3L��0.1000 mol/L��2.46��10-3mol
���Ը���ƷCuCl������Ϊ2.46��10-3mol��99.5g/mol��0.2448g
������Ʒ��CuCl������������ ��100%��97.91%��96.50%�����Ը���Ʒ��CuCl�������������ϱ���
��100%��97.91%��96.50%�����Ը���Ʒ��CuCl�������������ϱ���
��4��KSP [Fe(OH)3]��c(Fe3+)��c3(OH-)��4.0��10-38��c(H+)�� ����˷�ӦFe3����3 H2O
����˷�ӦFe3����3 H2O Fe(OH)3��3H����ƽ�ⳣ��K��
Fe(OH)3��3H����ƽ�ⳣ��K�� ��
�� ��2.5��10-5��
��2.5��10-5��
���㣺�����Լ���ѡ��Ӧ�����Ŀ�֪��������ѡ��������ԭ��Ӧ�͵���ƽ�ⳣ���ļ����Լ��йط���ʽ����д��

 ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�ѡ���������ʷ���ͼ��鷽��������ѡ�����ں����ϡ�
| A����ȡ�� | B�������� | C���ᾧ�� | D����Һ��E������F��������G������ |
��2��������غ��Ȼ��ƵĻ��Һ�л������� ��
��3������ˮ�����͵Ļ���� ��
��4������ƾ����е�Ϊ78.1�棩�ͼױ�(�е�Ϊ110.6��)���ֻ���Һ�� ��
(4��)����(����������)
| ��� | ���� | �����Լ� |
| ��1�� | CO2��HCl�� | |
| ��2�� | Fe2O3��Al2O3�� | |
| ��3�� | NaCl��Һ��MgCl2�� | |
| ��4�� | NO���壨NO2�� | |
�����е�Ħ����һ����CaCO3��Al(OH)3��SiO2��ɡ�ijС��Լ���������Ħ�����ɷּ��京������̽����
��1������������Ϣ���Ʋ�Ħ����Ӧ�߱���������
A��������ˮ B��������ˮ C�������ϴ� D��������С
��2����֪A�������Ħ������Al(OH)3��ȡ����������Ʒ����ˮ��ֽ��衢���ˣ�
���������м������NaOH��Һ��Al(OH)3��NaOH��Һ��Ӧ�����ӷ���ʽ____ __��
������������Һ��ͨ�����CO2���ټ������ϡ���ᣬ�۲쵽�������� ��
��3����֪B��������Ħ�������ܺ���һ���������ʣ����������Ħ������������ʱ�������в��������ʡ�
�� ��BƷ������Ħ�����ijɷ�����������裺
����1��ֻ��SiO2 ����2��������SiO2��CaCO3
����3��������________________��
�� ����Ʒ�����BƷ������Ħ�����ijɷֽ�����֤����д�±�����ѡ�Լ���ϡ���ᡢϡ���ᡢNaOH��Һ������ʯ��ˮ
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ����ˮ�ɷֽ��衢���ˣ�����ҺA�ͳ���B�� | |
| ����2�� | |
| ����3�� | |