��Ŀ����
18��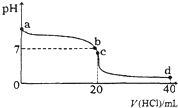 �����£���0.1mol•L-1�������20mL 0.1mol•L-1��ˮ�У���ҺpH�������������ı仯������ͼ��ʾ�������й�˵������ȷ���ǣ�������
�����£���0.1mol•L-1�������20mL 0.1mol•L-1��ˮ�У���ҺpH�������������ı仯������ͼ��ʾ�������й�˵������ȷ���ǣ�������| A�� | a���pH��7����Kw��Ϊ1.0��10-14 | |
| B�� | b����ʾ��Һ��C��Cl-��=C��NH4+�� | |
| C�� | C����ҺpH��7����ԭ����NH4++H2O?NH3•H2O+H+ | |
| D�� | d����ʾ��Һ������Ũ���ɴ�С�������ǣ�c��Cl-����C��NH4+����C��H+����C��OH-�� |
���� A��Kwֻ���¶ȵ�Ӱ�죻
B����b�㣬��Һ��pH=7��
C����c����Һ�У��������������Ϊ20mL���ܽ���ˮǡ����ȫ�кͣ�
D����d�㣬�������������Ϊ40mL��������������õ���ҺΪ��Ũ�ȵ�HCl��NH4Cl�Ļ���
��� �⣺A��Kwֻ���¶ȵ�Ӱ�죬��a����Һ��Kw��Ϊ1.0��10-14����A��ȷ��
B����b�㣬��Һ��pH=7������C��H+��=C��OH-�������ݵ���غ��֪��C��Cl-��=C��NH4+������B��ȷ��
C����c����Һ�У��������������Ϊ20mL���ܽ���ˮǡ����ȫ�кͣ������õ���ҺΪNH4Cl��Һ����ҺpH��7����ԭ����NH4+����Һ�л�ˮ�⣺NH4++H2O?NH3•H2O+H+����C��ȷ��
D����d�㣬�������������Ϊ40mL��������������õ���ҺΪ��Ũ�ȵ�HCl��NH4Cl�Ļ�����Һ�����ԣ�������NH4+ˮ�⣬��NH4+��Ũ��С��H+Ũ�ȣ���ȷ��˳��Ϊ��c��Cl-����C��H+����C��NH4+����C��OH-������D����
��ѡD��
���� ���⿼��������Һ�ͼ���Һ��Ϲ��̵�����Ũ�ȴ�С�Ƚϡ���Һ��Kwֵ�ı仯ֻ���¶ȵ�Ӱ������⣬�ѶȲ���ע������Ũ�ȴ�С�Ƚϵ����գ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
9��ij��ɫ��Һ���������÷ų�H2����������������п��ܵ��ǣ�������
| A�� | H+��Cl-��Cu2+��Ba2+ | B�� | OH-��NO${\;}_{3}^{-}$��Ba2+��Cl- | ||
| C�� | H+��CO${\;}_{3}^{2-}$��Mg2+��Ba2+ | D�� | OH-��NO${\;}_{3}^{-}$��CO${\;}_{3}^{2-}$��Mg2+ |
6����NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A�� | 1mol OH-���еĵ�����Ϊ9NA | |
| B�� | ���³�ѹ�£�NO2��N2O4�Ļ����23g�к���NA����ԭ�� | |
| C�� | ��״���£�2.8gN2��2.24L CO������������Ϊ1.4NA | |
| D�� | ��״���£�22.4 L �Ҵ��к���NA���Ҵ����� |
4������������Ҫ��FeS2��SiO2�ȣ��Ʊ�Ī���ε��������£�
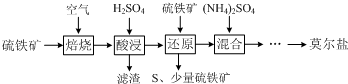
��֪������ԭ��ʱ��FeS2��H2SO4����Ӧ��Fe3+ͨ����Ӧ��ԭ�����з�Ӧ�����£�
2Fe3++FeS2=2S��+3Fe2+
��1������ԭ��ʱ��pH���˹��ߵ�ԭ����pH����ʱ��Ԫ�ؽ��������²��ʽ��ͣ�д������ԭ��ʱ��Ӧ������ӷ���ʽ��FeS2+14Fe3++8H2O=15Fe2++2SO42-+16H+��
��2��ʵ���á���ԭ��ʱ��Ӧ���б���ԭ��Fe3+�����ʵ���֮��Ϊ2��7�����㡰��ԭ������ҺFe2+��Ũ�ȼ���ȷ���������ӣ�NH4��2SO4��������Һ����仯���Բ��ƣ�
��3����ȡ23.52g����Ī���Σ�����ˮ�����Һ���ֳ����ȷݣ�һ�ݼ���������BaCl2��Һ���õ���ɫ����13.98g����һ����0.2000mol•L-1K2Cr2O7������Һ�ζ�����Cr2O72-ǡ����ȫ����ԭΪCr3+ʱ��������Һ�����Ϊ25.00mL����ȷ��Ī���εĻ�ѧʽ�������������̣���

��֪������ԭ��ʱ��FeS2��H2SO4����Ӧ��Fe3+ͨ����Ӧ��ԭ�����з�Ӧ�����£�
2Fe3++FeS2=2S��+3Fe2+
��1������ԭ��ʱ��pH���˹��ߵ�ԭ����pH����ʱ��Ԫ�ؽ��������²��ʽ��ͣ�д������ԭ��ʱ��Ӧ������ӷ���ʽ��FeS2+14Fe3++8H2O=15Fe2++2SO42-+16H+��
��2��ʵ���á���ԭ��ʱ��Ӧ���б���ԭ��Fe3+�����ʵ���֮��Ϊ2��7�����㡰��ԭ������ҺFe2+��Ũ�ȼ���ȷ���������ӣ�NH4��2SO4��������Һ����仯���Բ��ƣ�
| ���� | ����Ũ�ȣ�mol•L-1�� | |
| ��ԭǰ | ��ԭ�� | |
| SO42- | 3.20 | 3.50 |
| Fe2+ | 0.15 | 3.30 |
11�������ͬ������ʹ���������Һ��n��Cl-��=n��CH3COO-��=0.01mol����������������ǣ�������
| A�� | �ֱ�������CaCO3��Ӧʱ���ų���CO2һ���� | |
| B�� | ��NaOH��ȫ�к�ʱ�����������ĵ�NaOH�� | |
| C�� | ������Һ��pH��� | |
| D�� | ȡ����������CH3COOH��Һ���ֱ��ˮϡ��a����b������Һ��pH��ȣ���a��b |
8��֤��NaHSO3��Һ��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽���ǣ�������
| A�� | �������� | B�� | ����Ba��OH��2��Һ | C�� | �ⶨ��Һ��pHֵ | D�� | ����Ʒ����Һ |
9���£�N2H4���ǻ����������һ��ȼ�ϣ���ӦʱN2O4Ϊ����������Ӧ����N2��ˮ������
��֪��N2��g��+2O2��g���TN2O4��g������H=+a kJ/mol
N2H4��g��+O2��g���TN2��g��+2H2O��g������H=-b kJ/mol
���б�ʾ�º�N2O4��Ӧ���Ȼ�ѧ����ʽ����ȷ���ǣ�������
��֪��N2��g��+2O2��g���TN2O4��g������H=+a kJ/mol
N2H4��g��+O2��g���TN2��g��+2H2O��g������H=-b kJ/mol
���б�ʾ�º�N2O4��Ӧ���Ȼ�ѧ����ʽ����ȷ���ǣ�������
| A�� | 2N2H4��g��+N2O4��g���T3N2��g��+4H2O��g������H=-��b+a�� kJ/mol | |
| B�� | 2N2H4��g��+N2O4��g���T3N2��g��+4H2O��g������H=-��2b+a��kJ/mol | |
| C�� | 2N2H4��g��+N2O4��g���T3N2��g��+4H2O��g������H=-��a-2b�� kJ/mol | |
| D�� | 2N2H4��g��+N2O4��g���T3N2��g��+4H2O��g������H=-��b-a�� kJ/mol |
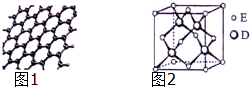 ԭ�������������������Ԫ��A��B��C��D��Eλ�����ڱ���ǰ�����ڣ�A��̬ԭ�ӵ�2p�������2��δ�ɶԵ��ӣ�C�������������Ǵ�����������3����C��Dͬ�������ڣ�Eλ�����ڱ���ds���������ֻ��һ�ԳɶԵ��ӣ���ش��������⣺
ԭ�������������������Ԫ��A��B��C��D��Eλ�����ڱ���ǰ�����ڣ�A��̬ԭ�ӵ�2p�������2��δ�ɶԵ��ӣ�C�������������Ǵ�����������3����C��Dͬ�������ڣ�Eλ�����ڱ���ds���������ֻ��һ�ԳɶԵ��ӣ���ش��������⣺