ЬтФПФкШн
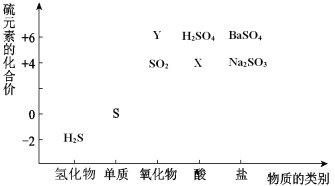
ЁОЬтФПЁПAЁЂBЁЂCЁЂD ЪЧдзгађЪ§вРДЮдіДѓЕФЭЌвЛЖЬЭЌЦкдЊЫиЃЌAЁЂB ЪЧН№ЪєдЊЫиЃЌCЁЂD ЪЧЗЧН№ЪєдЊЫиЃЌAЁЂBЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяПЩвдЗЂЩњЗДгІЩњГЩбЮКЭЫЎЁЃ
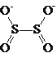
(1)A гы C ПЩаЮГЩЛЏКЯЮя A2CЃЌаДГіИУЛЏКЯЮяЕФЕчзгЪНЮЊ_____ЁЃ
(2)B гы D аЮГЩЕФЛЏКЯЮяЪЧ_____(ЬюЁАРызгЛЏКЯЮяЁБЛђЁАЙВМлЛЏКЯЮяЁБ)ЃЌбщжЄИУНс ТлЕФЪЕбщЗНЗЈЪЧ_____ЁЃ
(3)C ЕФЕЭМлбѕЛЏЮяЭЈШы D ЕЅжЪЕФЫЎШмвКжаЃЌЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_____ЁЃ
(4)гУ C ЕФзюИпМлКЌбѕЫс W ЕФШмвКзїЕчНтжЪШмвК(ЮяжЪЕФСПХЈЖШЮЊ 5.2mol/LЃЌЬхЛ§ЮЊ1LЃЌ МйЩшЗДгІЧАКѓШмвКЬхЛ§БфЛЏКіТдВЛМЦ)зщзАГЩдЕчГиШчЭМЫљЪОЁЃ

Ђйдк a ЕчМЋЩЯЗЂЩњЕФЗДгІПЩБэЪОЮЊ_____ЁЃ
ЂкШєЕчГиЙЄзївЛЖЮЪБМфКѓЃЌa МЋЯћКФ 0.05molPbЃЌ b ЕчМЋЕФжЪСПБфЛЏЮЊ________gЃЌдђДЫЪБ W ШмвКЕФХЈЖШ ЮЊ___________mol/LЁЃ
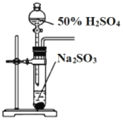
ЁОД№АИЁП![]() ЙВМлЛЏКЯЮя НЋИУЛЏКЯЮяМгШШжСШлШкзДЬЌзіЕМЕчадЪЕбщЃЌШчЙћИУЛЏКЯЮядкШлШкзДЬЌЯТВЛЕМЕчЃЌЫЕУїИУЛЏКЯЮяЪЧЙВМлЛЏКЯЮя SO2+Cl2+2H2O=H2SO4+2HCl Pb-2e-+SO42-=PbSO4 3.2 5.1
ЙВМлЛЏКЯЮя НЋИУЛЏКЯЮяМгШШжСШлШкзДЬЌзіЕМЕчадЪЕбщЃЌШчЙћИУЛЏКЯЮядкШлШкзДЬЌЯТВЛЕМЕчЃЌЫЕУїИУЛЏКЯЮяЪЧЙВМлЛЏКЯЮя SO2+Cl2+2H2O=H2SO4+2HCl Pb-2e-+SO42-=PbSO4 3.2 5.1
ЁОНтЮіЁП
AЁЂB ЪЧН№ЪєдЊЫиЃЌAЁЂBЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяПЩвдЗЂЩњЗДгІЩњГЩбЮКЭЫЎЃЌAЪЧNaдЊЫиЁЂBЪЧAlдЊЫиЃЛNaгы C ПЩаЮГЩЛЏКЯЮя A2CЃЌCЯд-2МлЃЌCЪЧSдЊЫиЃЛAЁЂBЁЂCЁЂD ЪЧдзгађЪ§вРДЮдіДѓЃЌЫљвдDЪЧClдЊЫиЁЃ
ИљОнвдЩЯЗжЮіЃЌ(1) AЪЧNaдЊЫиЁЂCЪЧSдЊЫиЃЌаЮГЩЛЏКЯЮяNa2SЪЧРызгЛЏКЯЮяЃЌЕчзгЪНЮЊ![]() ЃЛ
ЃЛ
(2) BЪЧAlдЊЫиЁЂ DЪЧClдЊЫиЃЌаЮГЩЕФЛЏКЯЮяAlCl3ЪЧЙВМлЛЏКЯЮяЃЌЙВМлЛЏКЯЮядкШлШкзДЬЌЯТВЛЕМЕчЃЌНЋИУЛЏКЯЮяМгШШжСШлШкзДЬЌзіЕМЕчадЪЕбщЃЌШчЙћИУЛЏКЯЮядкШлШкзДЬЌЯТВЛЕМЕчЃЌЫЕУїИУЛЏКЯЮяЪЧЙВМлЛЏКЯЮяЃЛ
(3)SЕФЕЭМлбѕЛЏЮяЪЧSO2ЃЌ D ЕЅжЪЪЧТШЦјЃЌSO2ЭЈШыТШЫЎЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊSO2+Cl2+2H2O=H2SO4+2HClЃЛ
(4) H2SO4ШмвКЁЂPbЁЂPbO2ЙЙГЩдЕчГиЃЌPbЪЧИКМЋЁЂPbO2ЪЧе§МЋЃЛ
Ђйa МЋЪЧИКМЋЃЌPbЪЇЕчзгЩњГЩPbSO4ГСЕэЃЌaЕчМЋЩЯЗЂЩњЕФЗДгІПЩБэЪОЮЊPb-2e-+SO42-=PbSO4ЃЛ
ЂкbЪЧе§МЋЃЌbЕчМЋЗДгІЪНЪЧPbO2+2e-+4H++SO42-=PbSO4+2H2OЃЛa МЋЯћКФ 0.05molPbЃЌзЊвЦЕчзгЕФЮяжЪЕФСПЪЧ0.1molЃЌ b ЕчМЋЯћКФ0.05mol PbO2ЃЌЩњГЩ0.05mol PbSO4жЪСПБфЛЏЮЊ![]() 3.2gЃЛИљОнзмЗДгІЪНPb+ PbO2+ H2SO4= 2PbSO4+2H2OЃЌa МЋЯћКФ 0.05molPbЃЌзмЗДгІЯћКФ0.1mol H2SO4ЃЌДЫЪБ H2SO4ШмвКЕФХЈЖШ ЮЊ
3.2gЃЛИљОнзмЗДгІЪНPb+ PbO2+ H2SO4= 2PbSO4+2H2OЃЌa МЋЯћКФ 0.05molPbЃЌзмЗДгІЯћКФ0.1mol H2SO4ЃЌДЫЪБ H2SO4ШмвКЕФХЈЖШ ЮЊ![]() =5.1mol/LЁЃ
=5.1mol/LЁЃ