��Ŀ����
10������һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��NH4+��Cl-��Mg2+��Ba2+��CO32-��SO42-��Ϊ��ȷ����Һ���������Ӽ������ʵ���Ũ�ȣ�ijͬѧ���ʵ�����£�ȡ����100mL����ˮ��Һ��������ʵ�飺�ٵ�һ�ݼ���AgNO3��Һ�г���������
�ڵڶ��ݼ�������NaOH��Һ�����ȣ��ռ�������896mL����״����
�۵����ݼ�������BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g����������ʵ�飬��ش�
��1����Һ�п϶����ڵ�������NH4+��SO42-��CO32-��K+����Щ���ӵ�Ũ����ȷ�����ֱ���NH4+��0.4mol/L��SO42-��0.1mol/L��CO32-��0.2mol/L��
��2���϶������ڵ�������Mg2+��Ba2+��������Mg2+��Ba2+��CO32-��Ba2+��SO42-���ܹ��棻
��3�����ܴ��ڵ�������Cl-�����ȷ�����ȼӹ���Ba��NO3��2��Һ��ȡ�ϲ���Һ�����ữ��AgNO3��Һ�����а�ɫ����˵����Cl-��������Cl-��
���� �ٵ�һ�ݼ���AgNO3��Һ�г���������˵�����ܺ���Cl-��CO32-��SO42-��
�ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.448L����״̬�£���֤��һ������NH4+�����ɵ�����Ϊ�������ʵ���=$\frac{0.896L}{22.4L/mol}$=0.04mol���������ɣ�����Һ��һ������Mg2+��Cu2+��
�۵����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g��֤��ԭ��Һ��һ������CO32-��SO42-������CO32-��SO42-��һ��������Ba2+��
���ɳ���BaSO4���ʵ���=$\frac{2.33g}{233g/mol}$�T0.01mol�����ɵij���BaCO3���ʵ���=$\frac{6.27g-2.33g}{197g/mol}$=0.02mol�����ݵ���غ㣬����K+��Cl-����ȷ�����ݴ˽��н��
��� �⣺��һ�ݣ���һ�ݼ���AgNO3����Һ�г���������˵����Һ�п��ܴ��ڣ�Cl-��CO32-��SO42-��
�ڶ��ݣ�������NaOH��Һ���Ⱥ����ɱ�״����0.896L����Ϊ����������Һ��һ������NH4+�������ʵ���Ϊ��$\frac{0.896L}{22.4L/mol}$=0.04mol��
�����ݣ�����������Ϣ��֪2.33Ϊ���ᱵ��n��BaSO4��=n��SO42-��=$\frac{2.33g}{233g/mol}$�T0.01mol�����ɵij���BaCO3���ʵ���=$\frac{6.27g-2.33g}{197g/mol}$=0.02mol����Ϊһ����CO32-���Բ���Mg2+���ٸ��ݵ���غ㣬�����Ϊ��n��+��=n��NH4+��=0.04mol��n��-��=2n��CO32-��+2n��SO42-��=0.06mol����һ����K+������0.02mol��
��1�����ݷ�����֪�϶����ڵ�������NH4+��SO42-��CO32-��K+���ܹ�ȷ��Ũ�ȵ�����ΪNH4+��SO42-��CO32-��
NH4+�����ʵ�����0.04mol����Һ�����0.1L������c��NH4+��=$\frac{0.04mol}{0.1L}$=0.4mol/L��SO42-���ʵ�����0.01mol����c��SO42-��=$\frac{0.01mol}{0.1L}$=0.1mol/L��CO32-�����ʵ�����0.02mol����c��CO32-��=$\frac{0.02mol}{0.1L}$=0.2mol/L��
�ʴ�Ϊ��NH4+��SO42-��CO32-��K+��NH4+��0.4mol/L��SO42-��0.1mol/L��CO32-��0.2mol/L��
��2������Mg2+��Ba2+��CO32-��Ba2+��SO42-������Ӧ���ɳ���������Һ��һ��������Mg2+��Ba2+��
�ʴ�Ϊ��Mg2+��Ba2+��Mg2+��Ba2+��CO32-��Ba2+��SO42-���ܹ��棻
��3�����ݷ�����֪����ȷ���Ƿ��������ӣ����ȼӹ���Ba��NO3��2��Һ����ȥ��������ӣ�Ȼ��ȡ�ϲ���Һ�����ữ��AgNO3��Һ�����а�ɫ����˵����Cl-��������Cl-��
�ʴ�Ϊ��Cl-���ȼӹ���Ba��NO3��2��Һ��ȡ�ϲ���Һ�����ữ��AgNO3��Һ�����а�ɫ����˵����Cl-��������Cl-��
���� ���⿼���˳������ӵļ��鷽������Ŀ�Ѷ��еȣ������漰�����ݵ���غ��ƶ����ӵĴ��ڣ�Ϊ�״��㣬ע���������ճ������ӵ����ʼ����鷽��������������ѧ���ķ���������������������


ҩƷ��������
| ����ȩ | ������ | ����� | ���� | |
| �ܽ�ȣ�25�棬g/100gˮ�� | 0.3 | ����ˮˮ�� | 0.04 | ���� |
| �е㣨�棩 | 179.6 | 138.6 | 300 | 118 |
| ��Է������� | 106 | 102 | 148 | 60 |
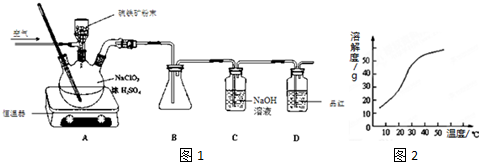
I���ϳɣ���Ӧװ����ͼ2��ʾ����������ƿ���Ⱥ������ϸ����ˮ�����ơ�4.8g����ȩ��5.6g����������ʹ֮��Ͼ��ȣ���150��170�����lСʱ��������״̬��
��1�����������ܵ�������ʹ��Ӧ������������
��2��Ϊ��ʹ�¶ȱ��ڿ��ƣ������Ⱦ��ȣ���װ��Ӧ���õļ��ȷ����ǿ���ԡ������ԡ�������Ȼ���Ҫ���Ʒ�Ӧ����״̬��������ҷ��ڣ��ᵼ���������ʽ��ͣ����ܵ�ԭ������������������Ӧ����٣�ƽ�����ƣ�
��3�������ô����ƾ��壨CH3COONa•3H2O����ԭ��������������ˮˮ�⣮
��Ʒ���ƣ���������Ӧ��õ��Ļ������ȵ���Բ����ƿ�У��������в�����
[��Ӧ�����]$��_{��������Һ}^{���뱥��}$ $��_{����ȩ}^{�����ȥ}$ $\stackrel{�����ữ}{��}$ $\stackrel{����1}{��}$ $��_{����}^{���ˡ�ϴ��}$[�����Ʒ�塿
��4���ӱ���Na2CO3��Һ����ת�����ᣬ����һ��Ŀ���ǽ������ת��Ϊ������ƣ��ܽ���ˮ��
��5������I����ȴ�ᾧ��
��6�����ʵ�鷽�������Ʒ���Ƿ��б���ȩȡ��������������Һ���ȣ������������֣�˵�����б���ȩ�������������������ͭ����Һ��������ש��ɫ������˵�����б���ȩ��
��7�������õ������������5.0g����÷�Ӧ�еIJ�����75%��������λ��Ч���֣���
 ��������֬������۷���Ϣ���ʵijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧװ��ʾ��ͼͼ1���й��������£�
��������֬������۷���Ϣ���ʵijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧװ��ʾ��ͼͼ1���й��������£�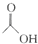 +
+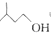 $?_{��}^{ŨH_{2}SO_{4}}$
$?_{��}^{ŨH_{2}SO_{4}}$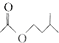 +H2O
+H2O| ��Է������� | �ܶ�/��g��cm-3�� | �е�/�� | ˮ���ܽ��� | |
| ���촼 | 88 | 0.8123 | 131 | �� |
| �Ҵ� | 60 | 1.0492 | 118 | �� |
| �Ҵ������� | 130 | 0.8670 | 142 | ���� |
��A�м���4.4g�����촼��6.0g�����ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50���ӣ���ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮ����þ���壬����Ƭ�̣����˳�ȥ����þ���壬�����������ռ�140��143����֣�������������3.9g���ش��������⣺

��1��װ��B�������ǣ�����������
��2����ϴ�Ӳ����У���һ��ˮϴ����ҪĿ���ǣ�ϴ��������ʹ�� �ڶ���ˮϴ����ҪĿ���ǣ�ϴ��̼������
��3����ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ��D�����ţ���
A��ֱ�ӽ������������ӷ�Һ©���Ͽڵ���
B��ֱ�ӽ������������ӷ�Һ©���¿ڷų�
C���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
D���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڷų�
��4����ʵ���м�����������Ŀ���ǣ���ߴ���ת����
��5��ʵ���м���������ˮ����þ��Ŀ���ǣ�����������������
��6������������У���ͼ2����ѡ��װ����ȷ���ǣ�b�����ţ�
��7����ʵ��IJ����ǣ�C
A��30% B��40% C��50% D��60%
��8���ڽ����������ʱ������130�濪ʼ�ռ���֣�����ƫ�ߣ�����ߵͣ�ԭ���ǻ��ռ�������δ��Ӧ�����촼��
| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| c��NO����mol/L�� | 1.00��10-3 | 4.50��10-4 | 2.50��10-4 | 1.50��10-4 | 1.00��10-4 | 1.00��10-4 |
| c��CO����mol/L�� | 3.60��10-3 | 3.05��10-3 | 2.85��10-3 | 2.75��10-3 | 2.70��10-3 | 2.70��10-3 |
��1�������������·�Ӧ�ܹ��Է����У���Ӧ�ġ�H��0����д������������������=������
��2��ǰ2s�ڵ�ƽ����Ӧ����v��N2��=1.88��10-4mol•L-1•s-1��
��3���ڸ��¶��£���Ӧ��ƽ�ⳣ��K=5000��
��4���������ܱ������з���������Ӧ���ﵽƽ��ʱ���д�ʩ�����NOת���ʵ���C��D��
A��ѡ�ø���Ч�Ĵ���B�����߷�Ӧ��ϵ���¶�
C�����ͷ�Ӧ��ϵ���¶�D����С���������
��5���о���������ʹ�õ���������ʱ����������ȱ���������ѧ��Ӧ���ʣ�Ϊ�˷ֱ���֤�¶ȡ������ȱ�����Ի�ѧ��Ӧ���ʵ�Ӱ����ɣ�ijͬѧ���������ʵ�飬����ʵ�������Ѿ���������ʵ����Ʊ��У�
| ʵ�� ��� | T���棩 | NO��ʼŨ�� ��mol/L�� | CO��ʼŨ�� ��mol/L�� | �����ıȱ������m2/g�� |
| �� | 280 | 1.20��10-3 | 5.80��10-3 | 82 |
| �� | 124 | |||
| �� | 350 | 124 |
| A�� | K+��Na+��SO42-��Mn2+ | B�� | Cl-��S2-��Fe2+��H+ | ||
| C�� | Na+��Ba2+��Br-��CO32- | D�� | Na+��H+��NO3-��HCO3- |
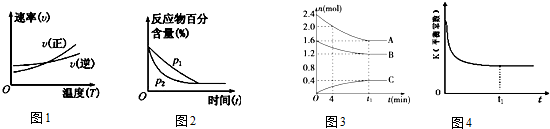 ��������
��������| A�� | ����ͼ�ٿ��ж�����Ӧ�ġ�H��0 | |
| B�� | ͼ�ڿɱ�ʾѹǿ��p���Է�Ӧ 2A��g��+2B��g��?3C��g��+D��s����Ӱ�죬P2 ��ѹǿ�� | |
| C�� | ͼ�ۿ��Ա�ʾ�ķ�ӦΪ 2A��g��+B��g��?C��g����H��0 | |
| D�� | ͼ�ܱ�ʾһ�������£��ھ��ȡ����ݵ��ܱ���ϵ�н��еķ�Ӧ��NO��g��+NO2��g��?N2O3��g����H��0��t1 ʱ�̴ﵽƽ��״̬ |