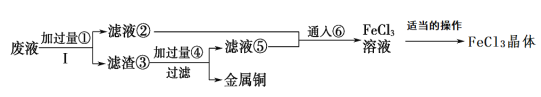
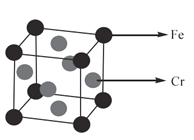
��Ŀ����
����Ŀ����ȩ��(OHC-COOH )��һ����Ҫ�ľ�ϸ������Ʒ���Ը߹�����Ϊ���Ե缫���ϣ��ú���Ƶ��NaBr ��Һ��������Ҷ�ȩ(OHC-CHO)�Ʊ���ȩ�OHC-CHO+Br2+H2O��OHC-COOH+2HBr��װ����ͼ��ʾ������˵������ȷ����( )
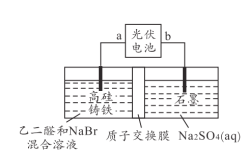
A.�缫bΪ�������߹����������
B.�����ĵ缫��ӦʽΪ2Br--2e-=Br2
C.�����ؽ��������ĵ���ת�Ƶ�ʯī��
D.�������У�������Һ��pH������
���𰸡�D
��������
�����Ҷ�ȩ(OHC-CHO)�Ʊ���ȩ���֪�߹�������������aΪ������bΪ���������������ӷŵ������嵥�ʣ������ǿ�����ԣ����Ҷ�ȩ����������ȩ�ᣬ����ˮ�������ӷŵ�����������
A.�ɷ�����֪�缫bΪ�������߹�����Ϊ���������������ӷŵ磬�߹���������ģ���A��ȷ��
B.�ɷ�����֪�����������ӷŵ磬�缫��ӦʽΪ2Br--2e-=Br2����B��ȷ��
C.����������������������������ص�������������ӵĸ���������������������ʯī����C��ȷ��
D.�������У�����Ϊˮ�е������ӷŵ磬ÿ����1mol��������2mol�����Ӵ������������ң���������������Һ������Ա��治�䣬��D����
������������ΪD��