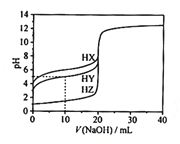
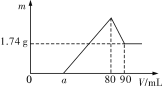
��Ŀ����
����Ŀ��һ��Ru�������g-C3N4���Ϲ������CO����ԭΪHCOOH��ԭ��ͼ��ͼ��
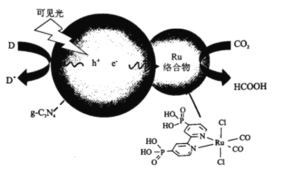
(1)��̬̼ԭ�ӵļ۵����Ų�ͼΪ___________��
(2)1molHCOOH�к��е�������ĿΪ___________��HCOOH�ķе��CO2�ߵ�ԭ��Ϊ___________��
(3)Ru������еڶ�����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ___________��
(4)Ru���������Ru��λ��ԭ����N��___________��
(5)һ��ʯī�ľۺ���뵼��g-C3N4���䵥��ƽ��ṹ��ͼ1�������ṹ��ͼ2��
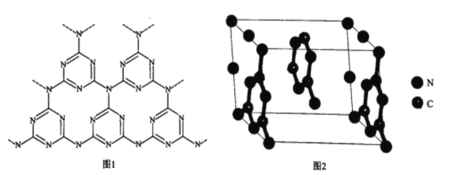
��g-C3N4�е�ԭ�ӵ��ӻ�������______________��
�ڸ���ͼ2����ͼ1����ƽ���ı��λ���һ����С�ظ���Ԫ��______________
����֪�þ��������ΪVcm3���м��ԭ�Ӿ��ھ����ڲ����谢���ӵ�������ֵΪNA����g-C3N4���ܶ�Ϊ______________g.cm-3��
���𰸡�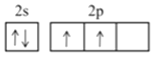 ��
�� 4NA HCOOH��CO2��Ϊ���Ӿ��壬HCOOH���Ӽ��γ���� N>O>C Cl��C sp2�ӻ�
4NA HCOOH��CO2��Ϊ���Ӿ��壬HCOOH���Ӽ��γ���� N>O>C Cl��C sp2�ӻ� 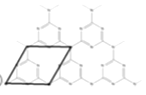
![]()
��������
�����������ԭ�����ɻ�̬̼ԭ�ӵļ۵����Ų�ʽȷ����۵����Ų�ͼ��HCOOH��CO2��Ϊ���Ӿ��壬HCOOH���Ӽ��γ�����������·е�ƫ�ߣ�ͬһ����Ԫ�صĵ�һ����������ԭ�������������������ͬһ���ڵĵڢ�AԪ�صĵ�һ�����ܴ��ڵڢ�A��ģ��ڢ�A��Ĵ��ڵڢ�A��ģ���ͼһ��֪N�γ�������ѧ������3���ӻ�������ӻ������ļн�Ϊ120����
(1)�����������ԭ������̬̼ԭ�ӵļ۵����Ų�ʽΪ2s22p2������۵����Ų�ͼΪ![]() ��
��![]() ��
��
(2)һ����������һ������,һ��˫���к���һ������,һ�����������ݼ�����ӽṹ��֪1���Ӽ����к�4����������1molHCOOH�к��е�������ĿΪ4NA��HCOOH��CO2��Ϊ���Ӿ��壬��HCOOH���Ӽ��γ���������������ͨ���Ӽ�������Ҫǿ����HCOOH�ķе��CO2�ߣ�
(3)Ru������к��еĵڶ�����Ԫ��ΪC��N��O��ͬһ����Ԫ�صĵ�һ����������ԭ�������������������ͬһ���ڵĵڢ�AԪ�صĵ�һ�����ܴ��ڵڢ�A��ģ��ڢ�A��Ĵ��ڵڢ�A��ģ��ʵ�һ�������ɴ�С��˳��ΪN>O>C��
(4)��Ru�����Ľṹ��֪��������Ru��λ��ԭ����N��Cl��C��
(5)����ͼһ��֪N�γ�������ѧ������3���ӻ�������ӻ������ļн�Ϊ120������Nԭ�ӵ��ӻ�����Ϊsp2�ӻ���
�ڸ���ͼ2����ͼ1����ƽ���ı��λ���һ����С�ظ���ԪΪ ��
��
����=m/V=M/VNA=![]() g.cm-3
g.cm-3

 ������������ϵ�д�
������������ϵ�д�����Ŀ���ҹ��Ǹ������������������Ϊ�����һ����¯��������Ϊ�ձ������������
I.��֪��Ӧ![]() Fe2O3(s)+CO(g)
Fe2O3(s)+CO(g)![]() Fe(s)+CO2(g)��H=-23.5kJmol-1���÷�Ӧ��1000����ƽ�ⳣ������4����һ���ݻ�Ϊ10L���ܱ������У�1000��ʱ����Fe��Fe2O3��CO��CO2��1.0mol��Ӧ����l0min��ﵽƽ�⡣
Fe(s)+CO2(g)��H=-23.5kJmol-1���÷�Ӧ��1000����ƽ�ⳣ������4����һ���ݻ�Ϊ10L���ܱ������У�1000��ʱ����Fe��Fe2O3��CO��CO2��1.0mol��Ӧ����l0min��ﵽƽ�⡣
(1)CO��ƽ��ת����=__��
(2)�����CO��ƽ��ת���ʣ��ٽ�Fe2O3��ת�����ɲ�ȡ�Ĵ�ʩ��__��
a.��߷�Ӧ�¶�
b.����Ӧ��ϵ��ѹǿ
c.ѡȡ���ʵĴ���
d.��ʱ���ջ��Ƴ�����CO2
e.�����ʯ��ʹ����ƽ���������ֽӴ�
��.��¯���������ķ����е�CO�ɽ��л��գ�ʹ����һ�������º�H2��Ӧ�Ʊ��״���CO(g)+2H2(g)![]() CH3OH(g)�������ͼʾ�ش��������⣺
CH3OH(g)�������ͼʾ�ش��������⣺
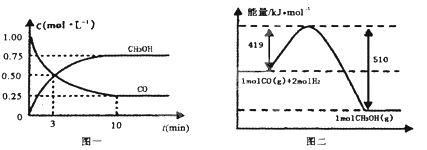
(3)�ӷ�Ӧ��ʼ��ƽ�⣬��H2Ũ�ȱ仯��ʾƽ����Ӧ����v(H2)=___��
(4)��֪������ȼ����286kJ/mol����д���״����岻���ȼ�յ��Ȼ�ѧ����ʽ___��
(5)�����¶Ⱥ�������ͬ�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ���÷�Ӧ�ﵽƽ�ⅼ���й��������±���
���� | ��Ӧ��Ͷ����� | ��Ӧ��� ת���� | CH3OH ��Ũ�� | �����仯(Q1��Q2��Q3������0) |
�� | 1molCO��2molH2 | ��1 | c1 | �ų�Q1kJ���� |
�� | 1molCH3OH | ��2 | c2 | ����Q2kJ���� |
�� | 2molCO��4molH2 | ��3 | c3 | �ų�Q3kJ���� |
�����й�ϵ��ȷ����___��
A.c1=c2 B.2Q1=Q3 C.2��1=��3 D.��/span>1+��2=1
E.�÷�Ӧ������1molCH3OH����ų�(Q1+Q2)kJ����
��.�Լ���Ϊȼ�ϵ����͵�أ���ɱ�����������Ϊȼ�ϵĴ�ͳȼ�ϵ�أ�Ŀǰ�õ��㷺���о�����ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ���ش��������⣺
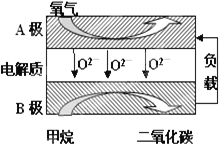
(6)B���ϵĵ缫��ӦʽΪ___��
(7)���ø�ȼ�ϵ������Դ����ʯī���缫���100mL1mol/L������ͭ��Һ���������ռ���������������ʱ�����������ĵļ�������Ϊ__(�����)��