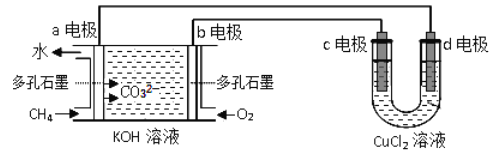
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΦν ΫΧΦΥαΆ≠[xCuCO3ΓΛyCu(OH)2]Θ§≥ ΩΉ»Η¬Χ―’…Ϊ.”÷≥ΤΈΣΩΉ»Η ·Θ§ «“Μ÷÷ΟϊΙσΒΡΩσΈο±Π ·ΓΘΥϋ”κΆ≠”κΩ’Τχ÷–ΒΡ―θΤχΓΔΕΰ―θΜ·ΧΦΚΆΥ°’τΤχΒ»Έο÷ Ζ¥”Π≤ζ…ζΒΡΈο÷ ΓΘCuSO4»ή“Κ”κNa2CO3»ή“ΚΖ¥”ΠΩ…“‘ΒΟΒΫΦν ΫΧΦΥαΆ≠Θ§Έ“Ο«ΫΪΕ‘ΤδΉι≥…Ϋχ––œύΙΊΧΫΨΩΓΘ
[≥ΝΒμ÷Τ±Η]
≥Τ»Γ12. 5 gΒ®Ζ·(CuSO4 5H2O)»ή”Ύ87. 5mL’τΝσΥ°÷–Θ§ΒΈΦ”…ΌΝΩœΓΝρΥα(ΧεΜΐΩ…“‘Κω¬‘≤ΜΦΤ)Θ§≥δΖ÷ΫΝΑηΚσΒΟΒΫCuSO4»ή“ΚΓΘœρΤδ÷–Φ”»κNa2CO3»ή“ΚΘ§ΫΪΥυΒΟάΕ¬Χ…Ϊ–ϋΉ«“ΚΙΐ¬ΥΘ§≤Δ”Ο’τΝσΥ°œ¥Β”Θ§‘Ό”ΟΈόΥ°““¥Φœ¥Β”Θ§ΉνΚσΒΆ€ΊΚφΗ…±Η”ΟΓΘ
[ Β―ιΧΫΨΩ]Έ“Ο«…ηΦΤΝΥ»γœ¬ΉΑ÷ΟΘ§”Ο÷ΤΒΟΒΡάΕ¬Χ…ΪΙΧΧεΫχ–– Β―ιΓΘ

ΗυΨί“‘…œ Β―ιΜΊ¥πœ¬Ν–Έ Χβ
(1)≈δ÷ΤΝρΥαΆ≠»ή“ΚΒΡΙΐ≥Χ÷–ΒΈΦ”œΓΝρΥαΒΡΉς”Ο «___________Θ§ΥυΒΟΝρΥαΆ≠»ή“ΚΒΡ»ή÷ ÷ ΝΩΖ÷ ΐΈΣ_________
(2) Β―ι “Ά®≥Θ Ι”ΟΦ”»»―«œθΥαΡΤΚΆ¬»Μ·οßΜλΚœ»ή“ΚΒΡΖΫΖ®÷Τ»ΓN2Θ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥ΧΈΣΘΚ__________ΓΘ
Δ«DΉΑ÷ΟΦ”»»«ΑΘ§–η“Σ Ήœ»¥ρΩΣΜν»ϊKΘ§Ά®»κ ΝΩN2Θ§»ΜΚσΙΊ±’KΘ§‘ΌΒψ»ΦD¥ΠΨΤΨΪΒΤΓΘΆ®»κN2ΒΡΉς
”Ο___________Θ§ BΈΣΑ≤»ΪΤΩΘ§ΤδΉς”Ο‘≠άμΈΣ_________Θ§C÷– ΔΉΑΒΡ ‘ΦΝ”Π «__________ΓΘ
(4)Φ”»»DΚσΙέ≤λΒΫΒΡœ÷œσ «________________ΓΘ
(5)Ψ≠≤ι‘ΡΈΡœΉ÷ΣΘΚKsp[CaCO3]=2.8ΓΝ10-9Θ§Ksp[BaCO3]=5.1ΓΝ10-9Θ§Ψ≠Χ÷¬έ»œΈΣ–η“Σ”ΟBa(OH)2»ή“Κ¥ζΧφ≥Έ«ε ·Μ“Υ°ά¥Ε®ΝΩ≤βΕ®άΕ¬Χ…ΪΙΧΧεΒΡΜ·―ß ΫΘ§Τδ‘≠“ρ «______________
a.Ba(OH)2ΒΡΦν–‘±»Ca(OH)2«Ω
b.Ba(OH)2»ήΫβΕ»¥σ”ΎCa(OH)2Θ§Ρή≥δΖ÷Έϋ ’CO2
c.œύΆ§ΧθΦΰœ¬Θ§CaCO3ΒΡ»ήΫβΕ»Οςœ‘¥σ”ΎBaCO3
d.Έϋ ’Β»ΝΩCO2…ζ≥…ΒΡBaCO3ΒΡ÷ ΝΩ¥σ”ΎCaCO3Θ§≤βΝΩΈσ≤ν–Γ
(6)¥ΐD÷–Ζ¥”ΠΆξ»ΪΚσΘ§¥ρΩΣΜν»ϊKΘ§‘Ό¥ΈΒΈΦ”NaNO2»ή“Κ≤ζ…ζN2Θ§ΤδΡΩΒΡ «______________ΓΘ»τΉΑ÷ΟF÷– Ι”ΟBa(OH)2»ή“ΚΘ§ Β―ιΫα χΚσΨ≠≥ΤΝΩΘ§ΉΑ÷ΟEΒΡ÷ ΝΩ‘ωΦ”0.27gΘ§F÷–≤ζ…ζ≥ΝΒμ1.97gΓΘ‘ρΗΟάΕ¬Χ…ΪΙΧΧεΒΡΜ·―ß ΫΈΣ_____________ΓΘ[–¥≥…xCuCO3ΓΛyCu(OH)2ΒΡ–Έ Ϋ]
ΓΨ¥πΑΗΓΩ “÷÷ΤCu2+Υ°ΫβΘ§Ζά÷Ι»ή“Κ±δΜκΉ« 8.0% NaNO2+NH4Cl![]() NaCl+N2Γϋ+2H2O ≈≈≥ΐΉΑ÷Ο÷–ΒΡΩ’Τχ Β±ΉΑ÷ΟΡΎ―ΙΝΠΙΐ¥σ ±Θ§BΤΩ÷–ΦδΒΡΑ≤»ΪΙή÷–“ΚΟφ…œ…ΐΘ§ Ι―ΙΝΠΈ»Ε® ≈®ΝρΥα ”≤÷ ≤ΘΝßΙή÷–άΕ¬Χ…ΪΙΧΧε±δΚΎ…ΪΘ§E÷–ΑΉ…ΪΙΧΧε±δάΕΘ§F÷–»ή“Κ±δΜκΉ« bd »ΟΆΘΝτ‘ΎΉΑ÷Ο÷–ΒΡΤχΧε±Μ≥δΖ÷Έϋ ’Θ§Φθ–Γ Β―ιΈσ≤ν 2CuCO3ΓΛ3Cu(OH)2
NaCl+N2Γϋ+2H2O ≈≈≥ΐΉΑ÷Ο÷–ΒΡΩ’Τχ Β±ΉΑ÷ΟΡΎ―ΙΝΠΙΐ¥σ ±Θ§BΤΩ÷–ΦδΒΡΑ≤»ΪΙή÷–“ΚΟφ…œ…ΐΘ§ Ι―ΙΝΠΈ»Ε® ≈®ΝρΥα ”≤÷ ≤ΘΝßΙή÷–άΕ¬Χ…ΪΙΧΧε±δΚΎ…ΪΘ§E÷–ΑΉ…ΪΙΧΧε±δάΕΘ§F÷–»ή“Κ±δΜκΉ« bd »ΟΆΘΝτ‘ΎΉΑ÷Ο÷–ΒΡΤχΧε±Μ≥δΖ÷Έϋ ’Θ§Φθ–Γ Β―ιΈσ≤ν 2CuCO3ΓΛ3Cu(OH)2
ΓΨΫβΈωΓΩ±Ψ ‘ΧβΩΦ≤ι Β―ι…ηΦΤΖΫΑΗΒΡΤάΦέΘ§Θ®1Θ©CuSO4 τ”Ύ«ΩΥα»θΦν―ΈΘ§»ή“Κ÷–¥φ‘ΎCu2ΘΪΘΪH2O ![]() Cu(OH)2ΘΪHΘΪΘ§ΒΈΦ”œΓΝρΥαΘ§HΘΪ≈®Ε»‘ω¥σΘ§“÷÷ΤCu2ΘΪΒΡΥ°ΫβΘ§“ρ¥ΥΒΈΦ”œΓΝρΥαΒΡΉς”Ο «“÷÷ΤCu2ΘΪΥ°ΫβΘ§Ζά÷Ι»ή“Κ±δΜκΉ«ΘΜ»ή÷ ΒΡ÷ ΝΩm(CuSO4)=12.5ΓΝ160/250g=8gΘ§»ή“ΚΒΡ÷ ΝΩΈΣ(12.5ΘΪ87.5)g=100gΘ§‘ρ÷ ΝΩΖ÷ ΐΈΣ8/100ΓΝ100%=8%ΘΜΘ®2Θ©NaNO2”κNH4ClΖΔ…ζ―θΜ·ΜΙ‘≠Ζ¥”ΠΘ§NaNO2ΘΪNH4ClΓζNaClΘΪN2ΓϋΘΪH2OΘ§NaNO2÷–NΜ·ΚœΦέ”…ΘΪ3ΦέΓζ0ΦέΘ§NH4Cl÷–NΒΡΜ·ΚœΦέ”…Θ≠3ΦέΓζ0ΦέΘ§ΗυΨίΜ·ΚœΦέΒΡ…ΐΫΒΖ®Ϋχ––≈δΤΫΘ§»ΜΚσΗυΨί‘≠Ή” ΊΚψ≈δΤΫΤδΥϊΘ§Φ¥Ζ¥”ΠΖΫ≥Χ ΫΈΣNaNO2+NH4Cl
Cu(OH)2ΘΪHΘΪΘ§ΒΈΦ”œΓΝρΥαΘ§HΘΪ≈®Ε»‘ω¥σΘ§“÷÷ΤCu2ΘΪΒΡΥ°ΫβΘ§“ρ¥ΥΒΈΦ”œΓΝρΥαΒΡΉς”Ο «“÷÷ΤCu2ΘΪΥ°ΫβΘ§Ζά÷Ι»ή“Κ±δΜκΉ«ΘΜ»ή÷ ΒΡ÷ ΝΩm(CuSO4)=12.5ΓΝ160/250g=8gΘ§»ή“ΚΒΡ÷ ΝΩΈΣ(12.5ΘΪ87.5)g=100gΘ§‘ρ÷ ΝΩΖ÷ ΐΈΣ8/100ΓΝ100%=8%ΘΜΘ®2Θ©NaNO2”κNH4ClΖΔ…ζ―θΜ·ΜΙ‘≠Ζ¥”ΠΘ§NaNO2ΘΪNH4ClΓζNaClΘΪN2ΓϋΘΪH2OΘ§NaNO2÷–NΜ·ΚœΦέ”…ΘΪ3ΦέΓζ0ΦέΘ§NH4Cl÷–NΒΡΜ·ΚœΦέ”…Θ≠3ΦέΓζ0ΦέΘ§ΗυΨίΜ·ΚœΦέΒΡ…ΐΫΒΖ®Ϋχ––≈δΤΫΘ§»ΜΚσΗυΨί‘≠Ή” ΊΚψ≈δΤΫΤδΥϊΘ§Φ¥Ζ¥”ΠΖΫ≥Χ ΫΈΣNaNO2+NH4Cl![]() NaCl+N2Γϋ+2H2OΘΜΘ®3Θ©Φ”»»«ΑΘ§Ά®»κN2ΒΡΡΩΒΡ «≈≈≥ΐΉΑ÷Ο÷–Ω’ΤχΘΜΉΑ÷ΟBΈΣΑ≤»ΪΤΩΘ§ΤδΉς”Ο‘≠άμ «Β±ΉΑ÷ΟΡΎ―ΙΝΠΙΐ¥σ ±Θ§BΤΩ÷–ΦδΒΡΑ≤»ΪΙή÷–“ΚΟφ…œ…ΐΘ§ Ι―ΙΝΠΈ»Ε®ΘΜΗυΨί Β―ιΡΩΒΡΘ§–η“Σ―ι÷ΛάΕ¬Χ…ΪΙΧΧε ή»»ΒΡ≤ζΈοΘ§“ρ¥ΥN2÷–ΒΡΥ°’τΤχΕ‘Κσ–χ Β―ι≤ζ…ζΗ…»≈Θ§±Ί–κ≥ΐ»ΞΘ§ΉΑ÷ΟCΒΡΉς”Ο «≥ΐ»ΞN2÷–ΒΡΥ°’τΤχΘ§Φ¥ΉΑ÷ΟC ΔΖ≈ΒΡ ‘ΦΝ «≈®ΝρΥαΘΜΘ®4Θ©≤ζΈο «CuOΓΔH2OΓΔCO2Θ§”≤÷ ≤ΘΝßΙή÷–άΕ¬Χ…ΪΙΧΧε±δΚΎ…ΪΘ§E÷–ΑΉ…ΪΙΧΧε±δάΕΘ§F÷–»ή“Κ±δΜκΉ«ΘΜΘ®5Θ©”ΟBa(OH)2»ή“Κ¥ζΧφCa(OH)2Θ§‘≠“ρ «ΘΚBa(OH)2ΒΡ»ήΫβΕ»¥σ”ΎCa(OH)2»ήΫβΕ»Θ§Ρή≥δΖ÷Έϋ ’CO2Θ§BaΒΡœύΕ‘‘≠Ή”÷ ΝΩ¥σ”ΎCaΒΡœύΕ‘‘≠Ή”÷ ΝΩΘ§Φ¥BaCO3ΒΡΡΠΕϊ÷ ΝΩ¥σ”ΎCaCO3ΒΡΡΠΕϊ÷ ΝΩΘ§Φθ…Ό≥ΤΝΩ ±≤ζ…ζΒΡΈσ≤νΘ§Ι ―Γœνbd’ΐ»ΖΘΜΘ®6Θ©Ζ¥”ΠΫα χΚσΘ§ΦΧ–χΆ®»κΒΣΤχΉς”Ο «»ΟΆΘΝτ‘ΎΉΑ÷Ο÷–ΒΡΤχΧε±Μ≥δΖ÷Έϋ ’Θ§Φθ–Γ Β―ιΈσ≤νΘΜΉΑ÷ΟE÷ ΝΩ‘ωΦ”ΒΡ «H2OΒΡ÷ ΝΩΘ§Φ¥n(H2O)=0.27/18mol=0.015molΘ§F÷–≥ΝΒμ «BaCO3Θ§Φ¥n(CO2)=1.97/197mol=0.01molΘ§H‘ΣΥΊά¥Ή‘”ΎCu(OH)2Θ§‘ρCu(OH)2ΒΡΈο÷ ΒΡΝΩΈΣ0.015molΘ§Cά¥Ή‘”ΎCuCO3Θ§‘ρCuCO3ΒΡΈο÷ ΒΡΝΩΈΣ0.01molΘ§x:y=0.01ΘΚ0.015=2ΘΚ3Θ§Φ¥2CuCO3ΓΛ3Cu(OH)2ΓΘ
NaCl+N2Γϋ+2H2OΘΜΘ®3Θ©Φ”»»«ΑΘ§Ά®»κN2ΒΡΡΩΒΡ «≈≈≥ΐΉΑ÷Ο÷–Ω’ΤχΘΜΉΑ÷ΟBΈΣΑ≤»ΪΤΩΘ§ΤδΉς”Ο‘≠άμ «Β±ΉΑ÷ΟΡΎ―ΙΝΠΙΐ¥σ ±Θ§BΤΩ÷–ΦδΒΡΑ≤»ΪΙή÷–“ΚΟφ…œ…ΐΘ§ Ι―ΙΝΠΈ»Ε®ΘΜΗυΨί Β―ιΡΩΒΡΘ§–η“Σ―ι÷ΛάΕ¬Χ…ΪΙΧΧε ή»»ΒΡ≤ζΈοΘ§“ρ¥ΥN2÷–ΒΡΥ°’τΤχΕ‘Κσ–χ Β―ι≤ζ…ζΗ…»≈Θ§±Ί–κ≥ΐ»ΞΘ§ΉΑ÷ΟCΒΡΉς”Ο «≥ΐ»ΞN2÷–ΒΡΥ°’τΤχΘ§Φ¥ΉΑ÷ΟC ΔΖ≈ΒΡ ‘ΦΝ «≈®ΝρΥαΘΜΘ®4Θ©≤ζΈο «CuOΓΔH2OΓΔCO2Θ§”≤÷ ≤ΘΝßΙή÷–άΕ¬Χ…ΪΙΧΧε±δΚΎ…ΪΘ§E÷–ΑΉ…ΪΙΧΧε±δάΕΘ§F÷–»ή“Κ±δΜκΉ«ΘΜΘ®5Θ©”ΟBa(OH)2»ή“Κ¥ζΧφCa(OH)2Θ§‘≠“ρ «ΘΚBa(OH)2ΒΡ»ήΫβΕ»¥σ”ΎCa(OH)2»ήΫβΕ»Θ§Ρή≥δΖ÷Έϋ ’CO2Θ§BaΒΡœύΕ‘‘≠Ή”÷ ΝΩ¥σ”ΎCaΒΡœύΕ‘‘≠Ή”÷ ΝΩΘ§Φ¥BaCO3ΒΡΡΠΕϊ÷ ΝΩ¥σ”ΎCaCO3ΒΡΡΠΕϊ÷ ΝΩΘ§Φθ…Ό≥ΤΝΩ ±≤ζ…ζΒΡΈσ≤νΘ§Ι ―Γœνbd’ΐ»ΖΘΜΘ®6Θ©Ζ¥”ΠΫα χΚσΘ§ΦΧ–χΆ®»κΒΣΤχΉς”Ο «»ΟΆΘΝτ‘ΎΉΑ÷Ο÷–ΒΡΤχΧε±Μ≥δΖ÷Έϋ ’Θ§Φθ–Γ Β―ιΈσ≤νΘΜΉΑ÷ΟE÷ ΝΩ‘ωΦ”ΒΡ «H2OΒΡ÷ ΝΩΘ§Φ¥n(H2O)=0.27/18mol=0.015molΘ§F÷–≥ΝΒμ «BaCO3Θ§Φ¥n(CO2)=1.97/197mol=0.01molΘ§H‘ΣΥΊά¥Ή‘”ΎCu(OH)2Θ§‘ρCu(OH)2ΒΡΈο÷ ΒΡΝΩΈΣ0.015molΘ§Cά¥Ή‘”ΎCuCO3Θ§‘ρCuCO3ΒΡΈο÷ ΒΡΝΩΈΣ0.01molΘ§x:y=0.01ΘΚ0.015=2ΘΚ3Θ§Φ¥2CuCO3ΓΛ3Cu(OH)2ΓΘ

 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
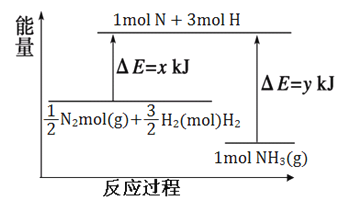
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ“―÷ΣH2(g)+Br2(l)=2HBr(g)ΘΜΓςH=-72kJ/molΘ°’τΖΔ1mol Br2(l)–η“ΣΈϋ ’ΒΡΡήΝΩΈΣ30kJΘ§ΤδΥϋœύΙΊ ΐΨί»γœ¬±μΘΚ
Μ·―ßΈο÷ | H2(g) | Br2(g) | HBr(g) |
1molΖ÷Ή”÷–ΒΡΜ·―ßΦϋΕœΝ― ±–η“ΣΈϋ ’ΒΡΡήΝΩ/KJ | 436 | a | 369 |
‘ρ±μ÷–aΈΣΘ®ΓΓΓΓΘ©
AΘ°404 BΘ°260 CΘ°230 DΘ°200