��Ŀ����
7��������֬�dz�Ĥ�����õ���֬����ͼΪһ�ִ�����֬�ĺϳ�·�ߣ�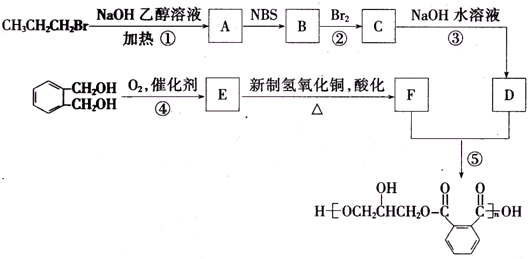
��֪��RCH2CH=CH2$\stackrel{NBS}{��}$
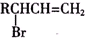
��1��B�к�̼�����ŵĽṹʽΪ
 ��C��������1��2��3-������飮
��C��������1��2��3-������飮��2�����顢1-������D�ķе��ɸߵ��͵�˳��ΪCH2OHCH��OH��CH2OH��CH3CH2CH2OH��CH3CH2CH2CH3�����ýṹ��ʽ��ʾ����
��3���ܵĻ�ѧ��Ӧ����ʽΪ
 ��
���ݵĻ�ѧ��Ӧ����ʽΪ
 ��
����4������������������
 ��ͬ���칹��Ľṹ��ʽ
��ͬ���칹��Ľṹ��ʽ ��
��a������FeCl3��Һ������ɫ��Ӧ
b��������һ�ȴ���������
c���ܷ�����ȥ��Ӧ��
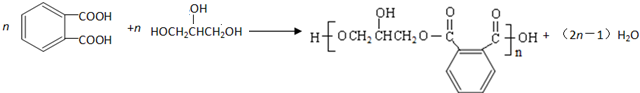
���� �����и����ʵ�ת����ϵ��֪��CH3CH2CH2Br�����������Ҵ���Һ�з�����ȥ��Ӧ����AΪCH3CH=CH2��CH3CH=CH2��NBS����֮������BΪBrCH2CH=CH2��BrCH2CH=CH2��Br2����CΪBrCH2CHBrCH2Br��BrCH2CHBrCH2Br�ټ���ˮ������DΪHOCH2CH��OH��CH2OH������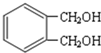 ��E��F��
��E��F��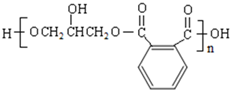 ����Ϸ�Ӧ������֪��EΪ
����Ϸ�Ӧ������֪��EΪ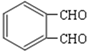 ��FΪ
��FΪ ���ݴ˴��⣮
���ݴ˴��⣮
��� �⣺�����и����ʵ�ת����ϵ��֪��CH3CH2CH2Br�����������Ҵ���Һ�з�����ȥ��Ӧ����AΪCH3CH=CH2��CH3CH=CH2��NBS����֮������BΪBrCH2CH=CH2��BrCH2CH=CH2��Br2����CΪBrCH2CHBrCH2Br��BrCH2CHBrCH2Br�ټ���ˮ������DΪHOCH2CH��OH��CH2OH������ ��E��F��
��E��F�� ����Ϸ�Ӧ������֪��EΪ
����Ϸ�Ӧ������֪��EΪ ��FΪ
��FΪ ��
��
��1��BΪBrCH2CH=CH2��B�к�̼�����ŵĽṹʽΪ ��CΪBrCH2CHBrCH2Br��C��������1��2��3-������飬
��CΪBrCH2CHBrCH2Br��C��������1��2��3-������飬
�ʴ�Ϊ�� ��1��2��3-������飻
��1��2��3-������飻
��2��DΪHOCH2CH��OH��CH2OH���ǻ�֮�����γ�������е�ϸߣ����Զ��顢1-������D�ķе��ɸߵ��͵�˳��Ϊ CH2OHCH��OH��CH2OH��CH3CH2CH2OH��CH3CH2CH2CH3��
�ʴ�Ϊ��CH2OHCH��OH��CH2OH��CH3CH2CH2OH��CH3CH2CH2CH3��
��3����Ӧ�ܵĻ�ѧ����ʽΪ��Ϊ ����Ӧ�ݵĻ�ѧ����ʽ��
����Ӧ�ݵĻ�ѧ����ʽ�� ��
��
�ʴ�Ϊ�� ��
�� ��
��
��4��������������a������FeCl3��Һ������ɫ��Ӧ��˵���зӵĽṹ��b��������һ�ȴ��������֣�c���ܷ�����ȥ��Ӧ��˵���ǻ���λ̼������ԭ�ӣ���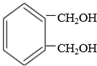 ��Ϊͬ���칹�壬�����Ľṹ��
��Ϊͬ���칹�壬�����Ľṹ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϣ���Ҫѧ���Ը������Ϣ�������ã��ܽϺõĿ���ѧ����ѧ������Ǩ������������ע�����ת����ϵ�е����ʽṹ�뷴Ӧ���������ƶϣ��������չ����ŵ����ʣ��Ѷ��еȣ�

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�| A�� | �����мӵ�ˮ����� | |
| B�� | ����������ˮ�У���ˮ��ӽ���ɫ | |
| C�� | ������ˮ���ܵõ������� | |
| D�� | ���ۺ���ά�ػ�Ϊͬ���칹�� |
| A�� | ��ϩ�ۺ�Ϊ����ϩ�߷��Ӳ��� | B�� | �ɱ��������� | ||
| C�� | ��ͭ��Ũ����Ϊԭ����������ͭ | D�� | ��SiO2�Ʊ��ߴ��� |
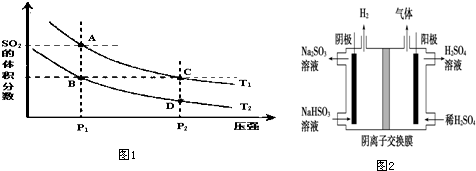
��1���Ƚ�Kֵ�Ĵ�С��K1��K2�����=��������
��2������˵����ȷ����CD
A��A��C���������Ӧ���ʣ�A��C
B��A��C����SO2�����Ũ�ȣ�A��C
C��B��C����������ƽ����Է���������B=C
D����״̬D��״̬C�������ü��ȵķ���
��3����500��ʱ���������ݻ����䣬ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����±��IJ������ݣ�
| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 |
| n��SO2��/mol | 2.00 | 0.60 | |||
| n��SO3��/mol | 0.00 | 0.80 | 1.8 | 1.8 |
�ڸ������·�Ӧ��ƽ�ⳣ��K��ֵΪ368.2����ȷ��С�����һλ����
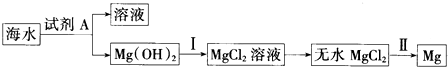
��4����ҵ����Na2SO3����β���е�SO2ʹ֮ת��ΪNaHSO3������ͼ2װ�õ�⣨���Ե缫��NaHSO3��ȡH2SO4���õ����������ܵ缫��Ӧʽ2HSO3-+2e-�TSO32-+H2���������ҳ����������⣬����������SO2 ��O2���ɣ������������ĸ���Ӧ�ĵ缫��ӦʽΪ2SO32-+4e-�T2SO2��+O2�����ù����п�ѭ�����õ�������Na2SO3��H2SO4��SO2���ѧʽ����
 ������˵������ȷ���ǣ�������
������˵������ȷ���ǣ�������| A�� | ���л���ķ���ʽΪC6H8O2�����ڷ����� | |
| B�� | ���л�������FeCl3��Һ������ɫ��Ӧ | |
| C�� | ���л���������ԭ�Ӳ�������ͬһƽ�� | |
| D�� | 1mol���л�����������Na��Ӧ�ɲ���1molH2 |
| A�� | ���ܺ���Ag+��Al3+��NH4+ | B�� | һ������Cl-�����ܺ���NO3- | ||
| C�� | һ������NH4+��AlO2-��CO32- | D�� | ���ܺ���Fe3+��һ������Fe2+ |
| A�� | v��A2��=0.8mol•L-1•s-1 | B�� | v��A2��=60mol•L-1•min-1 | ||
| C�� | v��AB3��=1.0mol•L-1•s-1 | D�� | v��B2��=1.2mol•L-1•s-1 |
| A�� | �Լ�AΪʯ���飬�۸�����������Ա��� | |
| B�� | �������м�����Լ�Ϊ���� | |
| C�� | ������ȡþ�Ĺ�����û���漰������ԭ��Ӧ | |
| D�� | �������������� |



 ���䷴Ӧ��������ȥ��Ӧ��
���䷴Ӧ��������ȥ��Ӧ�� ��
��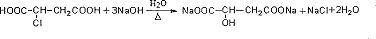 ��
�� ��
��