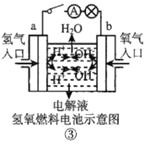
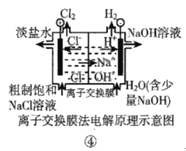
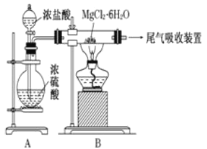
��Ŀ����
����Ŀ���о����������������ڴ����к������ӵ������ʱ���漰���·�Ӧ��
2NO2(g)��NaCl(s)![]() NaNO3(s)��ClNO(g)��K1����H1<0(��)
NaNO3(s)��ClNO(g)��K1����H1<0(��)
2NO(g)��Cl2(g)![]() 2ClNO(g)�� K2�� ��H2<0 (��)
2ClNO(g)�� K2�� ��H2<0 (��)
(1)4NO2(g)��2NaCl(s)![]() 2NaNO3(s)��2NO(g)��Cl2(g)��ƽ�ⳣ��K��________(��K1��K2��ʾ)��
2NaNO3(s)��2NO(g)��Cl2(g)��ƽ�ⳣ��K��________(��K1��K2��ʾ)��
(2)Ϊ�о���ͬ�����Է�Ӧ(��)��Ӱ�죬�ں��������£���2 L�����ܱ������м���0.2 mol NO��0.1 mol Cl2��10 minʱ��Ӧ(��)�ﵽƽ�⡣���ƽ���n(Cl2)��2.5��10��2 mol��10 min����(ClNO)��________________����NO��ת������1��________�������������ֲ��䣬��Ӧ(��)�ں�ѹ�����½��У�ƽ��ʱNO��ת������2 ________��1(����>������<����������)��
(3)�����ǹ�ҵ���������Ҫԭ��֮һ�������������з�������Ҫ��Ӧ���£�
I. 4NH3(g) + 5O2(g)![]() 4NO(g) + 6H2O(g) ��H =-906 kJ��mol��1
4NO(g) + 6H2O(g) ��H =-906 kJ��mol��1
II.4NH3(g) + 3O2(g) 2N2(g) + 6H2O(g) ��H = -1266 kJ��mol��1
��1L���ܱ������г���1 mol NH3��2 mol O2������й����ʵ����ʵ����뷴Ӧ�¶ȵĹ�ϵ��ͼ��ʾ��

�ٴ����������У������˵��¶�Ϊ_________________(����T1������T2�� ����T����)
��д��N2��O2����NO���Ȼ�ѧ����ʽ________��
���¶�ΪT2ʱ����ӦII��ƽ�ⳣ��K =_________________(ֻ����ʽ)
���𰸡�K12/K2 7.5��10��3 mol��L��1��min��1 75% > T�� N2(g)��O2(g)2NO(g) ��H= +180.5kJ��mol��1 0.22��0.96/0.44��1.453 mol��L��1
��������
��1���ֱ�д����������������Ӧ��ƽ�ⳣ��K1=c(ClNO)��c2(NO2)
K2= c2(ClNO)��[c2(NO)c(Cl2)]�����ݷ�Ӧ4NO2��g��+2NaCl��s��2NaNO3��s��+2NO��g��+Cl2��g����֪�÷�Ӧƽ�ⳣ��K=c(Cl2)��c2(NO)��[c4(NO2)]= K12/K2��
��2���ں��������£���2L�����ܱ������м���0.2mol NO��0.1mol Cl2��10minʱ��Ӧ���ﵽƽ�⣬���10min��v��ClNO��=7.5��10-3molL-1min-1�����ʵ���Ϊ7.5��10-3molL-1min-1��10min��2L=0.15mol��Ȼ������ƽ������ʽ��ʽ���㣻
(3)���������ﵪ����NO�����ʵ����жϴ����Է�Ӧ��ѡ���ԣ�
��Ӧ��Ϊ���ȷ�Ӧ����T��ʱ����Ӧ�ﵽƽ��״̬�����������¶ȣ�ƽ�������ƶ����Ӷ�ʹNO���ʵ������٣�
T2ƽ��ʱn(NO)��n(N2)��0.2mol�����÷���ʽ����������Ӧ���ĵİ������������ʵ����Լ����ɵ�ˮ�����ʵ��������Լ���ƽ��ʱ�������������ʵ����������������Ϊ1L���������ʵ�������Ũ�ȴ���K=c4(NO)c6(H2O)c4(NH3)c5(O2)���㣻
(1)( �ֱ�д��������Ӧ��ƽ�ⳣ��K1=c(ClNO)��c2(NO2)
K2= c2(ClNO)��[c2(NO)c(Cl2)]�����ݷ�Ӧ4NO2��g��+2NaCl��s��2NaNO3��s��+2NO��g��+Cl2��g����֪�÷�Ӧƽ�ⳣ��K=c(Cl2)��c2(NO)��[c4(NO2)]= K12/K2��
�ڶ��ַ�������Ӧ��=����2��,��K= K12/K2��
�ʴ�Ϊ��K12/K2��
(2)�ں���������,��2L�����ܱ������м���0.2molNO��0.1molCl2,10minʱ��Ӧ(��)�ﵽƽ��,���10min����(ClNO)= 7.5��10��3 mol��L��1��min��1,���ʵ���Ϊ7.5��103molL1min1��10min��2L=0.15mol����
2NO(g)��Cl2(g)![]() 2ClNO(g)��
2ClNO(g)��
��ʼ��(mol)0.2 0.1 0
�仯��(mol)0.15 0.075 0.15
ƽ����(mol)0.05 0.025 0.15
��ƽ���n(Cl2)=0.025mol��
NO��ת������1=0.15mol��0.2mol��100%=75%��
�����������ֲ���,��Ӧ(��)�ں�ѹ�����½���,�淴Ӧ����,���������С,Ϊ���ֺ�ѹ�������������С,ѹǿ����,ƽ���������,ƽ��ʱNO��ת������2����;
�ʴ�Ϊ��7.5��10��3 mol��L��1��min��1��75%��>��
(3) ����ͼ��֪���ô����ڸ���ʱ�����ɵ�NO���ʵ���Զ���ڵ����ģ��ʸô����ڸ�����ѡ��ӦI�������ڵ�����ѡ��Ӧ��Ӧ��Ϊ���ȷ�Ӧ����T3ʱ����Ӧ�ﵽƽ��״̬�����������¶ȣ�ƽ�������ƶ����Ӷ�ʹNO���ʵ������٣�
�ʴ�Ϊ��T��
���и�˹���ɵã���I- II����2����N2(g)��O2(g)2NO(g) ��H= +180.5kJ��mol��1
�ʴ�Ϊ��N2(g)��O2(g)2NO(g) ��H= +180.5kJ��mol��1
����1L�ܱ������г���span>1molNH3��2molO2��T2ƽ��ʱn(NO)��n(N2)��0.2mol����
4NH3(g)+5024NO(g)+6H2O(g)
�仯(mol)��0.2 0.25 0.2 0.3
4NH3(g)+302(g)2N2(g)+6H2O(g)
�仯(mol)��0.4 0.3 0.2 0.6
��ƽ��ʱ��n(NH3)��1mol0.2mol0.4mol��0.4mol��n(O2)��2mol0.25mol0.3mol��1.45mol��n(H2O)��0.3mol+0.6mol��0.9mol�������������Ϊ1L���������ʵ�������Ũ�ȼ���ƽ�ⳣ��K= K=c2(N2)c6(H2O)��(c4(NH3)c3(O2))= 0.22��0.96/0.44��1.453 mol��L��1
�ʴ�Ϊ��0.22��0.96/0.44��1.453 mol��L��1

 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�����Ŀ���� A �� B �� C �� D �� E �� F ����Ԫ�أ���֪��
�� ����λ��������ͬ�Ķ����ڣ��˵������������
��EԪ�صĵ��������ݼ��±���KJ/mol����
![]()
��B��Fͬ���塣
��A��E ������ D ��ԭ�Ӹ����� 1��1 �� 2��1 �γɻ����
��B��C ������ D ��ԭ�Ӹ����� 1:1 �� 1:2 �γɻ����
��1��д��ֻ���� A��B��D��E ����Ԫ�ص�������ˮ�εĻ�ѧʽ��_______________________________
��2��B2A2 �������______________ ��![]() ����_____________��
����_____________��![]() ����
����
��3������ͨ���Ѳ� 1mol ij��ѧ�������յ��������ɸû�ѧ���ļ��ܡ����ܵĴ�С���Ժ�����ѧ����ǿ����Ҳ�������ڼ��㻯ѧ��Ӧ�ķ�Ӧ��(��H)��ѧ��Ӧ�ķ�Ӧ�ȵ��ڷ�Ӧ�ж��Ѿɻ�ѧ���ļ���֮���뷴Ӧ���γ��»�ѧ���ļ���֮�͵IJ�±��г�����������Ԫ���γɵĻ�ѧ���ļ��ܣ�
��ѧ�� | F��D | F��F | B��B | F��B | B=D | D=D |
���ܣ�KJ/mol�� | 460 | 176 | 347.7 | 347 | 745 | 497.3 |
�Լ���1molF������ȫȼ��ʱ�ķ�Ӧ����H=________(��֪1molF�����к�2mol F��F����1molFO2�к���4mol��F��O)��