��Ŀ����
12��A��G�����ʼ�Ĺ�ϵ��ͼ������B��DΪ��̬���ʣ�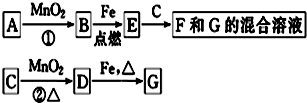
��ش��������⣮
��1������C��E�����Ʒֱ�ΪŨ���ᡢ������������
��2����ѡ�ò�ͬ��A���з�Ӧ�٣������ڳ����½��У��仯ѧ����ʽΪ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2������ֻ���ڼ�������½��У���Ӧ��AӦΪ�v�ѧʽ�wKClO3��
��3��MnO2�ڷ�Ӧ�ٺͷ�Ӧ���е����÷ֱ��Ǵ�������������
��4�������Ƶ�F��ҺӦ���������Է�ֹ��ת��ΪG������G��Һ�������ӵij����Լ���KSCN��Һ��ʵ������Ϊ��Һ��Ѫ��ɫ��
���� �����ͻ�Ƶ���MnO2������ѧ��ѧ��MnO2����ķ�Ӧ��Ҫ���������������Ʊ����������ݿ�ͼ��ת���ص��֪B��������D��������C��Ũ���ᣬE��������������F���Ȼ�������G���Ȼ�����Fe2+���л�ԭ�ԣ��������ʱ��Ҫ�������۷�ֹ�������������й�Ԫ�ػ�����֪ʶ���������غ�ĽǶ���д��ѧ����ʽ��
��� �⣺��1������ѧ��ѧ��MnO2����ķ�Ӧ��Ҫ���������������Ʊ����������ݿ�ͼ��ת���ص��֪B��������D��������C��Ũ���ᣬE��������������F���Ȼ�������G���Ȼ�����
�ʴ�Ϊ��Ũ���ᡢ������������
��2��MnO2�����Ʊ������ķ�Ӧ�����֣�һ������MnO2�������������£�����KClO3�ֽ��Ƶ�����������AΪKClO3����һ����MnO2�������������£�������H2O2�ֽ��Ƶ��������䷴Ӧ����ʽΪ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
�ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����KClO3��
��3����Ӧ��Ϊ�ڶ��������������������²�����������Ӧ��Ϊʵ������Ũ������������̼����Ʊ������ij��÷�������Ӧ�ж�����������������
�ʴ�Ϊ����������������
��4��Fe2+���л�ԭ�ԣ��ױ������е���������������������Ƶ��Ȼ�������Һ��Ҫ�������۷�ֹ��������Fe2+�ܱ����������Һ������ʹ���������Һ��ɫ�����Լ������Һ���Ƿ���Fe2+�IJ���Ϊȡ�����Һ������������������Һ������Һ��ɫ��ȥ��˵����Fe2+���ڣ�
�ʴ�Ϊ�����ۣ�KSCN��Һ����Һ��Ѫ��ɫ��
���� ������������ͼ�⣬��Ҫ���쳣�����ʵ����ʡ��Ʊ��ͼ��飬�����ɻ�ѧ�г����ķ�Ӧ���⣬ע��Ԫ�ػ�����֪ʶ�Ļ��ۺͳ������ӵļ��鷽����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 14.2 g | B�� | 16.7 g | C�� | 12.6g | D�� | 12 g |
 ��������������Ը��������Һ���õõ��������ֻ����CH3COCH3��CH3COCH2CH2COOH��CH3COOH����������B��A��Ϊͬ���칹�壬��A��B�ֱ������Ը��������Һ��Ӧ�õ��IJ�����ͬ������B�Ľṹ��ʽ��ȷ���ǣ�������
��������������Ը��������Һ���õõ��������ֻ����CH3COCH3��CH3COCH2CH2COOH��CH3COOH����������B��A��Ϊͬ���칹�壬��A��B�ֱ������Ը��������Һ��Ӧ�õ��IJ�����ͬ������B�Ľṹ��ʽ��ȷ���ǣ�������| A�� | CH3CH=C��CH3��-��CH2��2-C��CH3��=CHCH3 | B�� | �� CH3��2C=CH-��CH2��2-C��CH3��=CHCH3 | ||
| C�� | �� CH3��2C=C��CH3��-��CH2��2-CH=CHCH3 | D�� | �� CH3��2C=CH-��CH2��2-CH=C��CH3��2 |
| ���� �� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� | �� |
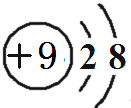 ��Ԫ�آٵ����������ĵ���ʽ��
��Ԫ�آٵ����������ĵ���ʽ�� ��
����2�������ĵڶ����ڵļ���Ԫ���У�ԭ�Ӱ뾶������C ����Ԫ�ط��ţ���
��3��Ԫ�آ��������������ˮ����ļ��Ը�ǿ��������NaOH��Ԫ�آ��������������ˮ����֮�����Ӧ�����ӷ���ʽΪAl��OH��3+OH-�TAlO2-+2H2O
��4��Ԫ�آ�������Թ��ۼ����ѧ�����ͣ���ϳ�ԭ�Ӿ��壨������ͣ���
| ������ | K+ Cu2+ Fe3+ Al3+ Fe2+ |
| ������ | Cl- CO32-NO3-SO42-SiO32- |
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ���棨����ɫ�ܲ����۲죩��
��ȡ������Һ������KSCN��Һ�����Ա仯��
����ȡ��Һ�����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ��������������ԭ��Һ��������ͬ��
������������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɣ�
���ƶϣ�
��1�����ɢ��жϣ���Һ��һ�������е���������K+��Fe3+��д���ӷ��ţ���
��2�����м�����������������ɫ��������ӷ���ʽ��3Fe2++NO3-+4H+�T3Fe3++NO��+2H2O��
��3�����������ú���ɫ����ͨ��ˮ�У��������ɫ���������Ļ�ѧ����ʽΪ3NO2+H2O�T2HNO3+NO��
��4����ͬѧ����ȷ��ԭ��Һ��������������Fe2+��Cu2+����������Cl-��NO3-��SO42-����д���ӷ��ţ�
��5����ҵ��ˮ�г����в�ͬ���͵���Ⱦ��ɲ��ò�ͬ�ķ�����������������ͬѧ��Ժ���ͬ��Ⱦ��ķ�ˮ����Ĵ�����ʩ�ͷ�����������ȷ����D��
| ѡ�� | ��Ⱦ�� | ������ʩ | ������� |
| A | ���� | ����ʯ���к� | ������ |
| B | Cu2+���ؽ������� | �������γ��� | ��ѧ�� |
| C | �������л���ķ�ˮ | ͨ�������л | ������ |
| D | ���Եķ�ˮ | ��CO2���к� | ��ѧ�� |
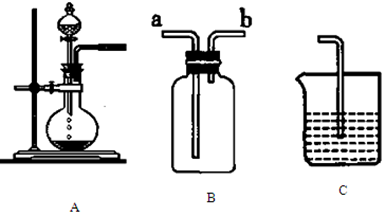
��1��װ��A�������Ʊ��������壬��д����Aװ���Ʊ���������ʱ��Բ����ƿ�ͷ�Һ©����Ӧװ�Ļ�ѧ�Լ�
| ���� | O2 | CO2 | HCl |
| �Լ� |
��3����Ҫ������װ����ȡCl2�������ʵ��Ƚ�Cl2��I2��������ǿ�������ڱ������������������װ�ú��Լ����ɲ�������
| װ�� | ��װ���з�Ӧ�����ӷ���ʽ |
| A�� | �����������������ʯӢ���ά | |
| B�� | �������ƿ����ڷ�ֹʳƷ�������ʣ��ӳ�ʳƷ�ı����� | |
| C�� | ̼�����������մ������������ͷۺ�����θ������ҩ�� | |
| D�� | ������������������ɫ�����Ϳ�� |
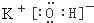 ��
�� ��
��