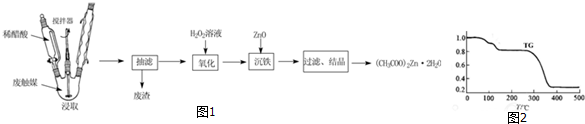
��Ŀ����
15�������������У��ٱ� ����Na2S���ۣ�NH4��2S����N2H4����C2H2 ��SiC����MgCl2 ��SiO2����NaOH����ɱ�������ţ���1������ֻ�������Ӽ��Ļ������Ǣڢߣ�
��2�����н����м��Թ��ۼ���ԭ�Ӿ����Ǣޢࣻ
��3�����к��м��Թ��ۼ������ӻ������Ǣۢ
��4�����к��зǼ��Թ��ۼ��Ĺ��ۻ������Ǣܢݣ�
��5�����к��з��»�����ֻ���м��Թ��ۼ��ķ��Ӿ����Ǣ٢⣮
���� ��1�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ����ǽ���Ԫ��֮�����γɹ��ۼ����������Ӽ��Ļ����������ӻ����
��2����ͬ�ǽ���Ԫ��֮����ڼ��Լ�����ԭ�ӹ��ɵľ�����ԭ�Ӿ��壻
��3��ͬ�ַǽ���Ԫ��֮����ڷǼ��Լ����������Ӽ��Ļ����������ӻ����
��4��ͬ�ַǽ���Ԫ��֮����ڷǼ��Լ���ֻ�����ۼ��Ļ������ǹ��ۻ����
��5����ͬ�ǽ���Ԫ��֮����ڼ��Լ������Ӿ����д��ڷ��»�����
��� �⣺��1�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ����ǽ���Ԫ��֮�����γɹ��ۼ����������Ӽ��Ļ����������ӻ����ֻ�����Ӽ��Ļ������Ǣڢߣ��ʴ�Ϊ���ڢߣ�
��2����ͬ�ǽ���Ԫ��֮����ڼ��Լ�����ԭ�ӹ��ɵľ�����ԭ�Ӿ��壬�����м��Թ��ۼ���ԭ�Ӿ����Ǣޢ࣬�ʴ�Ϊ���ޢࣻ
��3��ͬ�ַǽ���Ԫ��֮����ڷǼ��Լ����������Ӽ��Ļ����������ӻ�������м��Թ��ۼ������ӻ������Ǣۢᣬ�ʴ�Ϊ���ۢ
��4��ͬ�ַǽ���Ԫ��֮����ڷǼ��Լ���ֻ�����ۼ��Ļ������ǹ��ۻ�������зǼ��Թ��ۼ��Ĺ��ۻ������Ǣܢݣ��ʴ�Ϊ���ܢݣ�
��5����ͬ�ǽ���Ԫ��֮����ڼ��Լ������Ӿ����д��ڷ��»��������з��»�����ֻ���м��Թ��ۼ��ķ��Ӿ����Ǣ٢⣬�ʴ�Ϊ���٢⣮
���� ���⿼�黯ѧ���ͻ����������жϣ���ȷ���ʹ���������֮��������������ɽ��ע�ⲻ�ܸ����Ƿ��н���Ԫ���ж����ӻ����Ϊ�״��㣮

 ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�| A�� | ������Һ��pH=a��������Һ��ˮϡ�ͺ���Һ�и���Ũ�Ⱦ���С | |
| B�� | ��Ũ�Ⱦ�Ϊ0.01 mol•L-1��CH3COOH��CH3COONa��Һ�������Ϻ���Һ�У�c��CH3COOH��+c��CH3COO-��=0.01mol•L-1 | |
| C�� | ���������pHֵ��Ϊ3�Ĵ�������ᣬ�ֱ��ˮϡ����amL��bmL��ϡ�ͺ���Һ��pHֵ��Ϊ5����b��a=100 | |
| D�� | һ���¶��£���0.1mol•L-1�Ĵ�����Һ�м������ռ��Һ��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$�ı�ֵ���� |
| A�� | ��ɢ������ֱ������10-9��10-7m֮�� | |
| B�� | ���ܲ��������ЧӦ | |
| C�� | ���Ƕ����ڻ���� | |
| D�� | ��ɫ��ͬ |
| A�� | ���Թ��е�ͭ��Ũ������ȣ������Թܵײ��а�ɫ���岢������������ɫ���ʣ��˰�ɫ����Ϊ����ͭ����ɫ����Ϊ����ͭ | |
| B�� | Ũ�����ڹ��������±�ƣ�˵��Ũ����ȶ������ɵ���ɫ����������Ũ���� | |
| C�� | ��ͬ������ͭ�ֱ��������ĵ������Ũ�����ϡ���ᷴӦ��������Һ�ֱ�Ϊ��ɫ����ɫ�������ڷ�Ӧʱ������ͭ����Ũ��ǰ�ߴ����ں��� | |
| D�� | ��ͭƬ����Ũ�����У�������ʵ������˵��ͭ�����Ũ�����з����ۻ� |
��2��Ti��BH4��2��һ����Ҫ�Ĵ�����ϣ��ڻ�̬Ti2+�У�����ռ�ݵ�����ܲ����ΪM�����ܲ���е�ԭ�ӹ����Ϊ9��
��3��H2S��H2O2����Ҫ�������������ʾ��
| �۵�/K | �е�/K | ˮ�е��ܽ�ȣ���״���� | |
| H2S | 187 | 202 | 2.6 |
| H2O2 | 272 | 423 | ������Ȼ��� |
��4������������CO�����������ȣ�������ɫ�ӷ���Һ��Ni��CO��n����Ni��CO��n�����廥Ϊ�ȵ���������ӵĻ�ѧʽΪCN-��C22-��д��һ�����ɣ���
��5����֪CrO5��CrΪ+6�ۣ���CrO5�ĽṹʽΪ
 ��
�� | A�� | 4�ԣ�NH3��=5�ԣ�O2�� | B�� | 5�ԣ�O2��=6�ԣ�H2O�� | C�� | 2�ԣ�NH3��=3�ԣ�H2O�� | D�� | 4�ԣ�O2��=5�ԣ�NO�� |
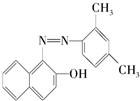 ��֪����˫��������Br2�����ӳɷ�Ӧ���յ����Ǻܶ���ҽ�ֹ����ʳƷ�����ĺϳ�ɫ�أ��ṹ��ʽ��ͼ�����й����յ����˵���д�����ǣ�������
��֪����˫��������Br2�����ӳɷ�Ӧ���յ����Ǻܶ���ҽ�ֹ����ʳƷ�����ĺϳ�ɫ�أ��ṹ��ʽ��ͼ�����й����յ����˵���д�����ǣ�������| A�� | �յ������ڷ����� | |
| B�� | �յ�������FeCl3��Һ������ɫ��Ӧ | |
| C�� | �յ����ܱ�����KMnO4��Һ���� | |
| D�� | 1 mol�յ���������1 mol Br2����ȡ����Ӧ |
| A�� | MgΪ��صĸ��� | B�� | ������ӦΪ��AgCl+e-�TAg+Cl- | ||
| C�� | ���ܱ�KCl ��Һ���� | D�� | �����ں���Ӧ���������� |