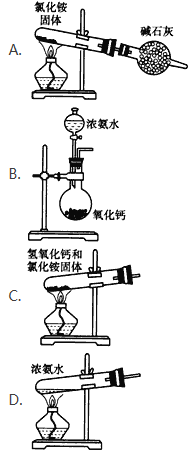
��Ŀ����
����Ŀ��ij������ˮ�к�����̬����ͨ������ʵ��ⶨ��Ũ����
��ȡˮ��10.0ml����ƿ������10��0mLKI��Һ(������������ָʾ��2-3�Ρ�
��ȡһ�ζ�������������ˮ������ˮϴ����Ȼ��ע��0��01mol��L��1��Na2S2O3��Һ������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2+2Na2S2O3=2NaI+Na2S4O6��
�Իش��������⣺
��1������ټ����ָʾ����______________��
��2�� �����Ӧʹ��___________ʽ�ζ��ܡ�
��3���������ζ��յ������___________________________��
��4������ȥNa2S2O3��Һ20��00mL�����ˮ��C12�����ʵ���Ũ��Ϊ______________��
��5����ָ��ʵ���е�һ�����Դ���_________________________��
���𰸡�(8����
��1��������Һ��
��2���
��3���������һ��Na2S2O3��Һ������Һǡ������ɫ��Ϊ��ɫ��30s���ٱ仯
��4��0.01mol��L-1
��5������ڵζ���������ˮϴ����δ�ô���Һ��ϴ
��������
�����������1����Һ���е��ʵ⣬���������Һ����ɫ�������������Ʒ���������ԭ��Ӧ������Ӧ�յ�ʱ����ɫ��ȥ���ʴ�Ϊ��������Һ��
��2�������������Һ�Լ��ԣ�Ӧѡ���ʽ�ζ��ܣ��ʴ�Ϊ���
��3���������۱���ɫ��������Һ����ɫ���淴ӦI2+2Na2S2O3=2NaI+2Na2S4O6���У���Һ��û�е⣬��Һ����ɫΪ��ɫ��˵����Ӧ���յ㣬�жϴﵽ�ζ��յ��ʵ�������ǣ������һ����Һ������ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ���ʴ�Ϊ�������һ����Һ������ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ��
��4��Cl2+2I-=2Cl-+I2��I2+2Na2S2O3=2NaI+Na2S4O6��
Cl2��2Na2S2O3
1 2
n 0.01molL-1��0.0200L
n=0.0001ml��c(Cl2��=![]() =0.01mol/L���ʴ�Ϊ��0.01mol/L��
=0.01mol/L���ʴ�Ϊ��0.01mol/L��
��5������ڵζ���������ˮϴ����Ӧ���ô���Һ��ϴ���ʴ�Ϊ������ڵζ���������ˮϴ����δ�ô���Һ��ϴ��

 ��ʱѵ���������������ϵ�д�
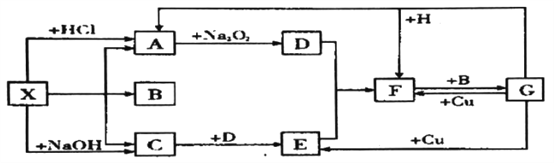
��ʱѵ���������������ϵ�д�����Ŀ�����в���Ԫ�ص�������ԭ�ӣ�����ӣ��ṹ���±���ʾ:
Ԫ�ر�� | Ԫ��������ԭ��(�����)�ṹ |
T | �����������Ǵ�����������3�� |
X | �����µ��ʷ���Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷ� |
Y | M���K����1������ |
Z | ��������Ԫ�صĽ��������а뾶��С |
U | ��������Ԫ�صķǽ������ӻ�ԭ������ |
��1��д��Ԫ��T����10�����ӵ�ԭ�ӷ��ţ�_________________��д��X��Y�γɵĻ�����ĵ���ʽ______________��
��2��Y��Z��ȣ������Խ�ǿ����________����Ԫ�ط��ţ������б���֤����һ��ʵ����__________������ĸ����
a��Y���ʵ��۵��Z���ʵ� b��Y������ˮ��Ӧ��Z������ˮ��Ӧ����
c��Y�Ļ��ϼ۱�Z�� d��Y����������Ӧ��ˮ����ļ��Ա�Z��ǿ
��3��д��Z��U����������Ӧ��ˮ�������Ӧ�ķ���ʽ____________________________
��4��M������T��Y��Z����Ԫ����ɣ���д����0.1 mol��M��Һ��60mL5 mol��L-1
�����ᷴӦ�����ӷ�Ӧʽ________________________________
��5��Ԫ��X����Ԫ����ԭ�Ӹ�����1��2�����γɳ����ڻ��ȼ�ϵĻ�����Ԫ��T����Ԫ����ԭ�Ӹ�����Ϊ1��1�����γɻ�����Q��Q��W����������ԭ��Ӧ����Ӧ�IJ��ﲻ��Ⱦ������д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________________��