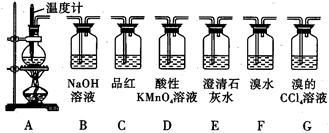
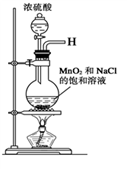
��Ŀ����
MnO2��һ����Ҫ�Ĵ�����ij�о���ѧϰС������˽���MnO2�����н϶��MnO��MnCO3����Ʒת��Ϊ��MnO2ʵ�飬���������£�
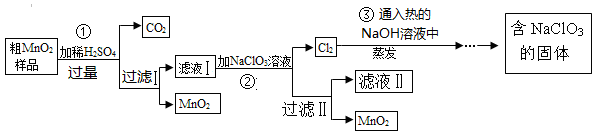
��1��д��1���ö��������������Ļ�ѧ��Ӧ����ʽ ��
��2���ڢڲ���Ӧ�����ӷ�Ӧ����ʽΪ ��
��3��������ˢ����õ�MnO2�Ƿ�ϴ�Ӹɾ��ķ����� ��
��4���ڢ۲���Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
��5�����������п�����ѭ��ʹ�õ������� ���ѧʽ�������˲��������������ж�Ҫ�õ��IJ��������� ��
��6������MnO2��Ʒ������Ϊ25.38g���ڢٲ���Ӧ�����˵õ�17.4g MnO2�����ռ���0.448LCO2����״���£�������Ʒ��������MnO����Ϊ g��

��1��д��1���ö��������������Ļ�ѧ��Ӧ����ʽ ��
��2���ڢڲ���Ӧ�����ӷ�Ӧ����ʽΪ ��
��3��������ˢ����õ�MnO2�Ƿ�ϴ�Ӹɾ��ķ����� ��
��4���ڢ۲���Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
��5�����������п�����ѭ��ʹ�õ������� ���ѧʽ�������˲��������������ж�Ҫ�õ��IJ��������� ��
��6������MnO2��Ʒ������Ϊ25.38g���ڢٲ���Ӧ�����˵õ�17.4g MnO2�����ռ���0.448LCO2����״���£�������Ʒ��������MnO����Ϊ g��
��1��2KClO3 2KCl��3O2����2H2O2
2KCl��3O2����2H2O2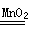 2H2O��O2��
2H2O��O2��
��2��5Mn2++2ClO3-+4H2O��Cl2��+5MnO2+8H+
��3��ȡ���һ��ϴ��Һ���Թ��У��μ��Ȼ�����Һ��������������֣�˵����ϴ�Ӹɾ�
��4��3Cl2+ 6NaOH NaClO3 + NaCl + 3H2O ��5��NaClO3������������1�֣� ��6��5. 68g
NaClO3 + NaCl + 3H2O ��5��NaClO3������������1�֣� ��6��5. 68g
 2KCl��3O2����2H2O2
2KCl��3O2����2H2O2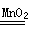 2H2O��O2��
2H2O��O2����2��5Mn2++2ClO3-+4H2O��Cl2��+5MnO2+8H+
��3��ȡ���һ��ϴ��Һ���Թ��У��μ��Ȼ�����Һ��������������֣�˵����ϴ�Ӹɾ�
��4��3Cl2+ 6NaOH
 NaClO3 + NaCl + 3H2O ��5��NaClO3������������1�֣� ��6��5. 68g
NaClO3 + NaCl + 3H2O ��5��NaClO3������������1�֣� ��6��5. 68g�����������1��ʵ��������ȡ����ʱ���ö�����������������Ӧ�Ļ�ѧ����ʽΪ2KClO3
 2KCl��3O2����2H2O2
2KCl��3O2����2H2O2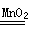 2H2O��O2����
2H2O��O2������2������ؾ���ǿ�����ԣ��ܰ���Һ�е�Mn2+�������ɶ������̣�������صĻ�ԭ��������������Ӧ�����ӷ���ʽΪ5Mn2++2ClO3-+4H2O��Cl2��+5MnO2+8H+��
��3����Һ�к���SO42��������������������SO42������˿���ͨ������SO42����������������Ƿ�ϴ�Ӹɾ���������ȷ��ʵ�������ȡ���һ��ϴ��Һ���Թ��У��μ��Ȼ�����Һ��������������֣�˵����ϴ�Ӹɾ���
��4���������ȵ�����������Һ��Ӧ���������ơ��Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ3Cl2+ 6NaOH
 NaClO3 + NaCl + 3H2O��
NaClO3 + NaCl + 3H2O����5���ɹ�������ͼ���Կ�����ѭ�����õ���NaClO3����ǰ����Ϊ��Ӧ����˺��������������������ʿ���ѭ��ʹ�á����˲��������������ж�Ҫ�õ��IJ��������Dz�������
��6����״����CO2�����ʵ�����0.448L��22.4L/mol��0.02mol�������̼ԭ���غ��֪̼���̵����ʵ�����0.02mol��������0.02mol��115g/mol��2.3g����ԭ�������MnO��������25.38g��17.4g��2.3g��5.68g��

��ϰ��ϵ�д�
�����Ŀ