��Ŀ����
����Ŀ����1�����з�Ӧ�����ڼ��˾���Ƿ�ƺ��ʻ��![]()
![]() ��
��
�������![]() ����
�У���![]() �γ���λ����ԭ����__________����Ԫ�ط��ţ���
�γ���λ����ԭ����__________����Ԫ�ط��ţ���
��![]() ��
��![]() ԭ�ӵ��ӻ����������________��
ԭ�ӵ��ӻ����������________��![]()
![]() ����
����![]() ������ĿΪ_______��
������ĿΪ_______��
��2��![]() ������
�����У�![]() ԭ�ӵ��ӻ����������__________��д����3��ԭ���������
ԭ�ӵ��ӻ����������__________��д����3��ԭ���������![]() ������ͬ�ռ乹�͵����ӣ�___________����һ�����ɣ���
������ͬ�ռ乹�͵����ӣ�___________����һ�����ɣ���
��3��ʯīϩ���ṹ��ͼ1��ʾ����һ���ɵ���̼ԭ�ӹ��ɵľ���ƽ��ṹ������̼���ϣ�ʯīϩ�в���̼ԭ�ӱ���������ƽ��ṹ�ᷢ���ı䣬ת��Ϊ����ʯīϩ���ṹ��ͼ2��ʾ����

����ʯīϩ��2��![]() ԭ�ӵ��ӻ���ʽ��_________����
ԭ�ӵ��ӻ���ʽ��_________����![]() ԭ��������
ԭ��������![]() ԭ���γɵļ���______������>����<������=����ʯīϩ��1��
ԭ���γɵļ���______������>����<������=����ʯīϩ��1��![]() ������
������![]() �γɵļ��ǡ�
�γɵļ��ǡ�
���𰸡�![]()
![]() �ӻ���
�ӻ���![]() �ӻ�
�ӻ� ![]()
![]() �ӻ�
�ӻ� ![]() ����
����![]() �ȣ�
�ȣ� ![]() �ӻ� <
�ӻ� <
��������
��1���������![]() ��
��![]() Ϊ�������ӣ�
Ϊ�������ӣ�![]() Ϊ���壬
Ϊ���壬![]() ԭ���ṩ�¶Ե��ӣ���
ԭ���ṩ�¶Ե��ӣ���![]() �γ���λ�����ʴ�Ϊ��
�γ���λ�����ʴ�Ϊ��![]() ��
��
��![]() ��
��![]() ԭ�ӷֱ��γ�4��
ԭ�ӷֱ��γ�4��![]() ����3��
����3��![]() ������û�й¶Ե��ӣ��ֱ�Ϊ
������û�й¶Ե��ӣ��ֱ�Ϊ![]() �ӻ���
�ӻ���![]() �ӻ�������
�ӻ�������![]() �Ľṹʽ��֪��
�Ľṹʽ��֪��![]() �����к���
�����к���![]() ������ĿΪ
������ĿΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() �ӻ���
�ӻ���![]() �ӻ���
�ӻ���![]() ��
��
��2����3��ԭ���������![]() ������ͬ�ռ乹�͵�������
������ͬ�ռ乹�͵�������![]() ��Ϊ�ȵ����壬��������
��Ϊ�ȵ����壬��������![]() ��
��![]() �ȣ��ʴ�Ϊ��
�ȣ��ʴ�Ϊ��![]() �ӻ���
�ӻ���![]() ����
����![]() �ȣ���
�ȣ���
��3������ʯīϩ��2��![]() ԭ���γ�3��
ԭ���γ�3��![]() ����1��
����1��![]() ����
����![]() ԭ�Ӳ�ȡ
ԭ�Ӳ�ȡ![]() �ӻ�����
�ӻ�����![]() ԭ�Ӻ�����������4��ԭ���γ������壬��ʯīϩ�е�1��
ԭ�Ӻ�����������4��ԭ���γ������壬��ʯīϩ�е�1��![]() ԭ���γ�3��
ԭ���γ�3��![]() ����ͼ1Ϊƽ��ṹ��
����ͼ1Ϊƽ��ṹ��![]() ԭ�Ӳ�ȡ
ԭ�Ӳ�ȡ![]() �ӻ�����
�ӻ�����![]() ԭ�Ӻ�����������3��ԭ���γ�ƽ�������Σ�������ʯīϩ��2��
ԭ�Ӻ�����������3��ԭ���γ�ƽ�������Σ�������ʯīϩ��2��![]() ԭ��������
ԭ��������![]() ԭ���γɵļ���<ʯīϩ��1��
ԭ���γɵļ���<ʯīϩ��1��![]() ԭ��������
ԭ��������![]() ԭ���γɵļ��ǣ��ʴ�Ϊ��
ԭ���γɵļ��ǣ��ʴ�Ϊ��![]() �ӻ���<��
�ӻ���<��

����Ŀ��1��2-���ȱ���(CH2ClCHClCH3)��һ����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͽ��ñ�ϩ�ӳɷ��Ʊ�����Ҫ������Ϊ3-�ȱ�ϩ(CH2=CHCH2Cl)����Ӧԭ��Ϊ��
I��CH2=CHCH3(g)+Cl2(g) ![]() CH2ClCHClCH3(g) H1=-134kJ��mol-1
CH2ClCHClCH3(g) H1=-134kJ��mol-1
II��CH2=CHCH3(g)+Cl2(g) CH2=CHCH2Cl(g)+HCl(g) H2=-102kJ��mol-1
��ش��������⣺
(1)��֪CH2=CHCH2Cl(g)+HCl(g) ![]() CH2ClCHClCH3(g)�Ļ��Ea(��)Ϊ132kJ��mol-1����÷�Ӧ�Ļ��Ea(��)Ϊ___________kJ��mol-1��
CH2ClCHClCH3(g)�Ļ��Ea(��)Ϊ132kJ��mol-1����÷�Ӧ�Ļ��Ea(��)Ϊ___________kJ��mol-1��
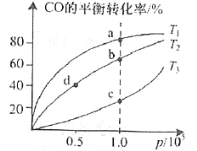
(2)һ���¶��£��ܱ������з�����ӦI�ͷ�ӦII���ﵽƽ�������ѹǿ��CH2ClCHClCH3�IJ���____________���������С�����䡱����������_________________________________��
(3)��ʼʱ��ij���ݾ��������г���1 mol CH2=CHCH3��1 mol Cl2������ӦII���ﵽƽ��ʱ������������ѹǿ_________________���������С�����䡱����
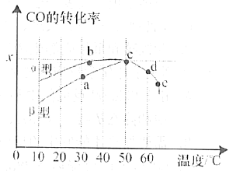
(4)ij�о�С�����ܱ������г���һ������CH2=CHCH3��Cl2���ֱ���A��B���ֲ�ͬ���������·�����Ӧ��һ��ʱ�����CH2ClCHClCH3�IJ������¶ȵĹ�ϵ��ͼ��ʾ��p���Ƿ�Ϊ��Ӧ�¶���CH2ClCHClCH3��ƽ����ʣ�_________��ǡ����ж�������_______________________��

(5)һ���¶��£�������ܱ������г�������ʵ�����CH2=CHCH3(g)��Cl2(g)���ڴ��������·�����ӦI�������������ѹǿ��ʱ��ı仯���±���ʾ��
ʱ��/min | 0 | 60 | 120 | 180 | 240 | 300 | 360 |
ѹǿ/kPa | 80 | 74.2 | 69.4 | 65.2 | 61.6 | 57.6 | 57.6 |
���õ�λʱ���������ѹ�ı仯����ʾ��Ӧ���ʣ���![]()
�ڸ��¶��£���ƽ��ʱHCl���������Ϊ![]() �����ϩ��ƽ����ת����
�����ϩ��ƽ����ת����![]() ____________����ӦI��ƽ�ⳣ��Kp=____________________kPa-1(KpΪ�Է�ѹ��ʾ��ƽ�ⳣ��������С�����2λ)��
____________����ӦI��ƽ�ⳣ��Kp=____________________kPa-1(KpΪ�Է�ѹ��ʾ��ƽ�ⳣ��������С�����2λ)��
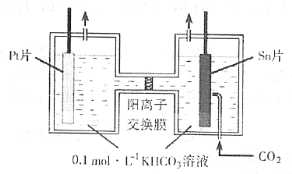
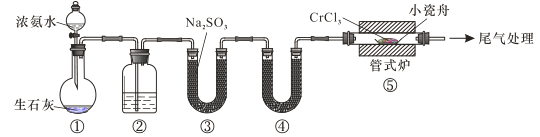
����Ŀ����ͼ�ǵ¹���ѧ�����ϣ1831��ⶨ������ֻ��C��H����Ԫ�أ���ɵ�װ�á������ڵ�����Ʒ�����ȷֽ���������ô�![]() �����Ͼ������ȵ�
�����Ͼ������ȵ�![]() ������������ﷴӦ���
������������ﷴӦ���![]() ��
��![]() �������չ����ա�
�������չ����ա�

��֪�±����ݣ�
���� | ���չܢ� | ���չܢ� | |||
���� | ��Ʒ+���� | ����ǰ | ���պ� | ����ǰ | ���պ� |
A | B | C | D | E | F |
(1)����Ʒ�ڷ�Ӧ���������õ���������________________________��
(2)���չܢ�Ӧװ�����ռ���____________�����չܢ�Ӧװ�����ռ���____________��
(3)��Ʒ��̼��������������ѧ����ʽ��________________________��
(4)ȡ����![]() �������г��ȼ�պ�����
�������г��ȼ�պ�����![]() ��
��![]() ����������һ��������������������ȡ����Ӧ������һ�ȴ���ֻ��һ�֣�����A�Ľṹ��ʽΪ___________����ϵͳ����������Ϊ____________��
����������һ��������������������ȡ����Ӧ������һ�ȴ���ֻ��һ�֣�����A�Ľṹ��ʽΪ___________����ϵͳ����������Ϊ____________��
����Ŀ��������Ԫ�����ڱ���һ���֣��ش������й����⣺
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | �� | �� | �� | |
4 | �� |
(1)д������Ԫ�ط��ţ���_______����_______����_______��_________��
(2)����ЩԪ���γɵĵ����У�����õĽ���������_____________������õķǽ���������_________���û�ѧ�������𣩡�
(3)����ЩԪ�ص�����������Ӧˮ�����У�������ǿ���ǣ��ѧʽ����ͬ��____________��������ǿ����____________�������Ե���___________����������֮�����Ӧ�Ļ�ѧ����ʽ��________________________��________________________��________________________
(4)�ڢ�����γɵĵ����У���ѧ���ʽϻ��õ���____________�������ƣ����������ԭ����ͬ�ļ�ʵ�顣
����һ��________________________________________________��
��������________________________________________________��
(5)�ڢ�����γɵĵ����У���ѧ���ʽϻ��õ���____________��д������֤���ý��۵�һ�����ӷ�Ӧ����ʽ________________________��