��Ŀ����
����Ŀ��ĿǰHaber-Bosch���ǹ�ҵ�ϳɰ�����Ҫ��ʽ��������������Ҫ���¸�ѹ��Ϊ����Ч�����ܺģ����ɽ�������ԭ�����ϳɰ�����Ϊ�Ǿ��о�ǰ�������������������һ�������������롪��Ӧ���Ѹ��ȹ��̣�ͼʾΪN2��H2�ڹ����������ϳɰ���Ӧ·����������ͼ(����������)�����С�*����ʾ������������

(1)�������Ѹ���____����(����ȡ����ȡ�)���ϳɰ����Ȼ�ѧ����ʽΪ_____
(2)�ϳɰ��Ľ�ķ������ȷ����ʷ���ʽΪ w= k1 p(N2)![]() -k2
-k2![]() ��wΪ��Ӧ��˲ʱ�����ʣ�Ϊ����Ӧ���淴Ӧ����֮�k1��k2�������淴Ӧ���ʳ������ϳɰ���ӦN2+3H22NH3��ƽ�ⳣ��Kp=_________(��k1��k2��ʾ)(ע��Kp�ø�����ƽ���ѹ����ʾ)��
��wΪ��Ӧ��˲ʱ�����ʣ�Ϊ����Ӧ���淴Ӧ����֮�k1��k2�������淴Ӧ���ʳ������ϳɰ���ӦN2+3H22NH3��ƽ�ⳣ��Kp=_________(��k1��k2��ʾ)(ע��Kp�ø�����ƽ���ѹ����ʾ)��
(3)����2.0molN2��6.0molH2ͨ�����Ϊ1L���ܱ������У��ֱ���T1��T2�¶��½��з�Ӧ������A��ʾT2�¶���n(H2)�ı仯������B��ʾT1�¶���n(NH3)�ı仯��T2�¶��·�Ӧ��a��ǡ�ôﵽƽ�⡣
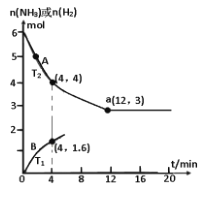
���¶�T1___T2 (�������������������ͬ)��T1�¶���ǡ��ƽ��ʱ������B�ϵĵ�Ϊb(m�� n )����m___12��n__2��
��T2 �¶��£���Ӧ�ӿ�ʼ��ǡ��ƽ��ʱƽ������v(N2) =_____��
��T2�¶��£��ϳɰ���ӦN2+3H22NH3��ƽ�ⳣ������ֵ��____����ijʱ�̣������������ѹǿΪ��ʼʱ��80%�����ʱv(��)____v(��)(�����������������)��
(4)��ҵ��ͨ�����ͷ�Ӧ����������¶ȶ�ʹ����������������ַ������ʵķ����� ��ԭ�����������з����е�___(�����)��
A.���� B.���� C.���� D.��ȡ
���𰸡����� N2(g)+3H2(g) 2NH3(g) ��H= -92 kJ��mol-1 ![]() �� �� �� 0.083molL-1min-1 0.148 �� B
�� �� �� 0.083molL-1min-1 0.148 �� B
��������
(1)��������ͼ��֪�������Ӵ���������ʱ�����������ߣ�Ϊ���ȹ��̣���ͼ��֪0.5mol������1.5mol����ת���1mol�����ķ�Ӧ��Ϊ��21 kJ��mol-1-17kJ��mol-1-50 kJ��mol-1=-46 kJ��mol-1����ϳɰ����Ȼ�ѧ����ʽΪN2(g)+3H2(g) 2NH3(g) ��H= -92 kJ��mol-1��
�ʴ�Ϊ�����ȣ�N2(g)+3H2(g) 2NH3(g) ��H= -92 kJ��mol-1��
(2)��Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ���w = k1 p(N2)![]() -k2
-k2![]() =0��k1 p(N2)
=0��k1 p(N2)![]() =k2
=k2![]() �������ã�
�������ã�![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(3) ������A��ʾT2�¶���n(H2)�ı仯����Ӧ��4minʱ�� ���������¶�Ӧ����������Ϊ��
���������¶�Ӧ����������Ϊ��![]() ������B��ʾT1�¶���n(NH3)�ı仯��4minʱ
������B��ʾT1�¶���n(NH3)�ı仯��4minʱ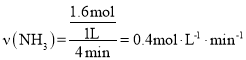 ������B��Ӧ�ķ�Ӧ���ʿ죬��֪T1��T2��������B��Ӧ���¶ȸߣ����ʿ죬���Ե���ƽ���ʱ�������A�̣���m��12������ӦΪ���ȷ�Ӧ���¶�����ƽ�������ƶ�����������Bƽ��ʱ���������ʵ���������Aƽ��ʱ���������ʵ����٣���n��2,
������B��Ӧ�ķ�Ӧ���ʿ죬��֪T1��T2��������B��Ӧ���¶ȸߣ����ʿ죬���Ե���ƽ���ʱ�������A�̣���m��12������ӦΪ���ȷ�Ӧ���¶�����ƽ�������ƶ�����������Bƽ��ʱ���������ʵ���������Aƽ��ʱ���������ʵ����٣���n��2,
�ʴ�Ϊ��������������
��T2 �¶��£���Ӧ�ﵽƽ��ʱ�� ��v(N2) =
��v(N2) =![]() ��
��
�ʴ�Ϊ��0.083molL-1min-1��
��T2�¶��£��ϳɰ���ӦN2+3H22NH3��������������������ʽ��

ƽ�ⳣ��K=![]() ��
��
�����ϼ����֪ƽ��ʱ��������Ϊ6mol��ƽ��ʱ�����ѹǿ����ʼʱ��![]() ����ijʱ�̣������������ѹǿΪ��ʼʱ��80%����ӦӦ�����������С�ķ�����ƽ�⼴��������У���v(��)��v(��)���ʴ�Ϊ��0.148������
����ijʱ�̣������������ѹǿΪ��ʼʱ��80%����ӦӦ�����������С�ķ�����ƽ�⼴��������У���v(��)��v(��)���ʴ�Ϊ��0.148������
(4)��ҵ��ͨ�����ͷ�Ӧ����������¶ȶ�ʹ����Һ��������������������������۷е���죬������ת��Һ���뵪���������������÷������������ʴ�Ϊ��B��

 ����ѧ���ʱѧ����ϵ�д�
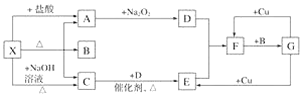
����ѧ���ʱѧ����ϵ�д�����Ŀ��ʵ�����о�ijЩ��������ʿ�����ͼ��ʾװ�ã����й��̺ͽ��۾���ȷ����

��ѡ | X | Y | Z | ���� |
A | Br2 | �� | NaOH ��Һ | �����巢��ȡ����Ӧ |
B | SO2 | ����KMnO4��Һ | NaOH ��Һ | �����������Ư���� |
C | HCl | Na2SiO3��Һ | NaCl ��Һ | Cl �ķǽ�����ǿ��Si |
D | CH2= CH2 | Br2 ��CCl4��Һ | AgNO3��Һ | ��ϩ��Br2�����ӳɷ�Ӧ |
A.AB.BC.CD.D
����Ŀ����Ԫ�غ�±��Ԫ�ض����γɶ������ʣ����ǿ���������ѧ���ʽṹ�����ʵ����֪ʶȥ��ʶ�����⡣
��1��COCl2�Ŀռ乹��Ϊ______________����Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ_____________��
��2����֪CsICl2���ȶ��������ֽ⣬���������ɾ����ܸ�������ʣ�����������_____________������ĸ��ʽ������
A.CsICl2====CsCl+ICl B.CsICl2====CsI+Cl2
��3�����ݱ��ṩ�ĵ�һ�����������жϣ����п������ɽ��ȶ��ĵ��������ӵ�±��ԭ����____��
Ԫ�� | �� | �� | �� | �� |
��һ������/ ��kJ��mol-1�� | 1681 | 1251 | 1140 | 1008 |
��4�����з��ӼȲ����ڡ�s-p���Ҽ���Ҳ�����ڡ�p-p���м�����__________������ĸ����
A.HCl B.HF C.SO2 D.SCl2
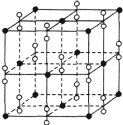
��5����֪ClO2��Ϊ��V���Σ�������ԭ����Χ��4�Լ۲���ӡ�ClO2��������ԭ�ӵ��ӻ��������Ϊ______________��д��һ����CN����Ϊ�ȵ���������ʵķ���ʽ��______________��
��6������������ȼ��ʱ�õ�һ�ָƵ������ᄃ�壬��ṹ��ͼ��ʾ���ɴ˿��жϸƵ�������Ļ�ѧʽΪ__________����֪����������ܶ��Ǧ�g��cm-3�����������������������ӵļ��Ϊ_________cm��ֻҪ������ʽ�����ؼ������ֵ�������ӵ�������ֵΪNA����