题目内容
【题目】高锰酸钾(KMnO4)和双氧水(H2O2)都是常见物质。
(1)写出二者共同元素的原子最外层轨道表示式:___________K在周期表的位置是 ___________ ;25Mn属于_____________族元素。
(2)双氧水(H2O2)和水都是极弱电解质,但H2O2比H2O更显酸性。若把H2O2看成是二元弱酸,请写出它在水中的电离方程式:_____________
(3)2g高锰酸钾溶于10ml水时溶液没有明显的温度变化,请解释理由__________________________________,氧化剂一般来说,酸性越强其氧化性也越强,因此常用酸化的高锰酸钾作氧化剂。用高锰酸钾法测定H2O2时,不能用HCl来酸化原因是_________________;
(4)往H2O2水溶液中滴入一滴酸性KMnO4溶液,从溶液内部析出大量无色气泡。写出可能的化学反应方程式。_____________________________________
(5)往H2O2水溶液中滴入酸性KMnO4溶液,下列方程式错误的是__________
A 3H2O2+2KMnO4+3H2SO4→K2SO4+2MnSO4+6H2O+4O2↑
B 5H2O2+2KMnO4+3H2SO4→K2SO4+2MnSO4+8H2O+5O2↑
C 7H2O2+2KMnO4+3H2SO4→K2SO4+2MnSO4+10H2O+6O2↑
D 9H2O2+2KMnO4+3H2SO4→K2SO4+2MnSO4+12H2O+7O2↑
【答案】![]() 第四周期ⅠA ⅦB或副族 H2O2
第四周期ⅠA ⅦB或副族 H2O2 ![]() H+ + HO2-, HO2-
H+ + HO2-, HO2- ![]() H+ + O22- 高锰酸钾溶于水时扩散过程吸收的能量与水合过程放出的能量相近 因HCl具有还原性 5H2O2+2KMnO4+3H2SO4 → K2SO4+2MnSO4+8H2O+5O2↑,2 H2O2
H+ + O22- 高锰酸钾溶于水时扩散过程吸收的能量与水合过程放出的能量相近 因HCl具有还原性 5H2O2+2KMnO4+3H2SO4 → K2SO4+2MnSO4+8H2O+5O2↑,2 H2O2 ![]() 2H2O + O2↑ A
2H2O + O2↑ A
【解析】
(1)二者共同元素为氧元素,O原子最外层轨道表示式为:![]() ;K在第四个周期,最外层一个电子,所以在周期表中的位置是第四周期IA族;25Mn属于VIIB族元素,故答案为:
;K在第四个周期,最外层一个电子,所以在周期表中的位置是第四周期IA族;25Mn属于VIIB族元素,故答案为:![]() ;第四周期IA族;VIIB或副族;
;第四周期IA族;VIIB或副族;
(2)多元弱酸分步电离,以第一步为主,所以电离方程式为:H2O2![]() H+ + HO2-,HO2-
H+ + HO2-,HO2-![]() H++O22-;答案为:H2O2
H++O22-;答案为:H2O2![]() H+ + HO2-,HO2-
H+ + HO2-,HO2-![]() H++O22-;
H++O22-;
(3)没有明显的温度变化,说明高锰酸钾溶于水扩散过程吸收的能量与水合过程放出的能量相近或相等,高锰酸钾强氧化性,HCl具有还原性,会发生氧化还原,答案为:高锰酸钾溶于水时扩散过程吸收的能量与水合过程放出的能量相近;因HCl具有还原性;
(4)往H2O2水溶液中滴入一滴酸性KMnO4溶液,从溶液内部析出大量无色气泡,说明产生氧气,可能是两者反应产生,或者过氧化氢自身分解产生氧气;方程式为:5H2O2+2KMnO4+3H2SO4=K2SO4+2MnSO4+8H2O+5O2↑,2 H2O2 ![]() 2H2O + O2↑;
2H2O + O2↑;
(5)根据得失电子守恒和原子守恒判断,A错误。

 新思维假期作业寒假吉林大学出版社系列答案
新思维假期作业寒假吉林大学出版社系列答案【题目】下表记录了t℃时的4份相同的硫酸铜溶液中加入无水硫酸铜的质量以及析出的硫酸 铜晶体(CuSO4·5H2O)的质量(温度保持不变)的实验数椐:
硫酸铜溶液 | ① | ② | ③ | ④ |
加入的无水硫酸铜(g) | 3.00 | 5.50 | 8.50 | 10.00 |
析出的硫酸铜晶体(g) | 1.00 | 5.50 | 10.90 | 13.60 |
当加入6.20g无水硫酸铜时,析出硫酸铜晶体的质量(g)为
A.7.70B.6.76C.5.85D.9.00
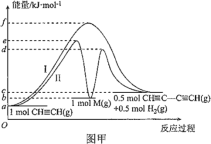
【题目】中国研究人员研制出一种新型复合光催化剂,利用太阳光在催化剂表面实现高效分解水,其主要过程如下图所示。

已知:几种物质中化学键的键能如下表所示。
化学键 | H2O中H—O键 | O2中O=O 键 | H2中H—H键 | H2O2中O—O键 | H2O2中O—H键 |
键能kJ/mol | 463 | 496 | 436 | 138 | 463 |
若反应过程中分解了2 mol水,则下列说法不正确的是
A. 总反应为2H2O![]() 2H2↑+O2↑
2H2↑+O2↑
B. 过程I吸收了926 kJ能量
C. 过程II放出了574 kJ能量
D. 过程Ⅲ属于放热反应