��Ŀ����
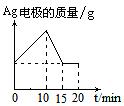
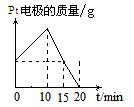
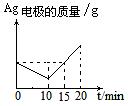
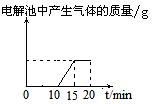
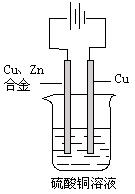
��10�֣����ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ������

��1����X��п��Y��̼���缫��a�DZ���NaCI��Һ����ٸ�ԭ�����Ƶû���ԭ���� ����Y���ĵ缫��ӦʽΪ
��2����Ҫ�õ�ⷽ��������ͭ����ٵ��Һaѡ�� ����Y�缫��Ӧʽ�� ����˵�������ʷ����ĵ缫��Ӧ����д����
��3����Ҫ��ij����С��Ʒ�϶���һ���������Y�缫�IJ����� ����X�缫��Ӧʽ�� ��

��1����X��п��Y��̼���缫��a�DZ���NaCI��Һ����ٸ�ԭ�����Ƶû���ԭ���� ����Y���ĵ缫��ӦʽΪ
��2����Ҫ�õ�ⷽ��������ͭ����ٵ��Һaѡ�� ����Y�缫��Ӧʽ�� ����˵�������ʷ����ĵ缫��Ӧ����д����
��3����Ҫ��ij����С��Ʒ�϶���һ���������Y�缫�IJ����� ����X�缫��Ӧʽ�� ��
��1�����������������������ƣ�2�֣���2Cl��+2e=Cl2��2�֣�
��2����CuSO4��Һ��1�֣� ��Cu-2e��= Cu2+��2�֣�
��3��������1�֣� ��Ag++e��= Ag��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ

 �� ��
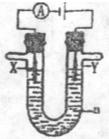
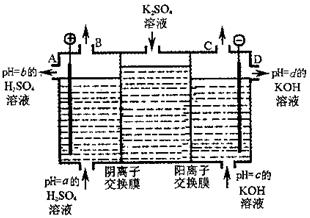
�� �� ����ʾ�����ձ�����
����ʾ�����ձ����� ����ʾ���������缫�����ֱ���M��N�����е绯ѧʵ��̽����
����ʾ���������缫�����ֱ���M��N�����е绯ѧʵ��̽���� ��ش��������⣺
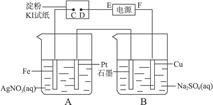
��ش��������⣺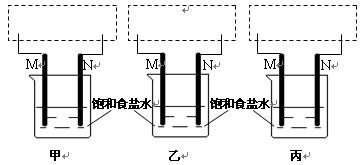
 ��
��
 ����(14��)
����(14��)