��Ŀ����
ijʵ��С�����ñ���ʳ��ˮ�����ߡ�ֱ����Դ���á� �� ��
�� �� ����ʾ�����ձ�����
����ʾ�����ձ�����
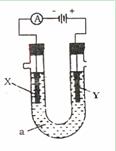
�������ƣ��á� ����ʾ���������缫�����ֱ���M��N�����е绯ѧʵ��̽����
����ʾ���������缫�����ֱ���M��N�����е绯ѧʵ��̽����
��ͬѧ��װ���������Ӻ�ֱ����Դͨ�缸���ӣ�����M����Һ��dz��ɫ����һ��ʱ�䣬��Һ��û��������ֺ���ɫ��
��ͬѧ���õ������ͼ�ͬѧ�Ŀ���ȥ��ͬ�����Ӻ�ֱ����Դͨ�缸���ӣ�ȴ�ŵ�һ�ɴ̱ǵ���ζ������ֹͣͨ�硣
��ͬѧ��װ����������·�պϼ����Ӻ�ȴû�з�������������������ֺܿ�������������ƣ����ֵ����Ƶ�ָ�뷢����ƫת��
���������ͬѧ��ʵ���� ��ش��������⣺
��ش��������⣺
��1��M�缫�������ǣ�д��ѧʽ�� ��N�缫�������ǣ�д��ѧʽ�� ��
��2���������������ɶ�Ӧ����ͬѧ��װ��ͼ��
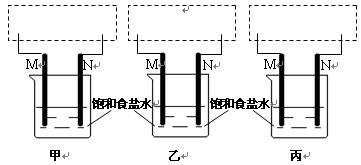
��3����Ҫ��д������ͬѧʵ��������漰�ķ�Ӧ����ʽ��
��ͬѧN�缫����ʽ
��ͬѧ�ܷ�Ӧ�����ӷ���ʽ
��ͬѧN�缫����ʽ
��4���û�ѧ����ʽ���ͼ�ͬѧʵ��ʱ�۲쵽M����Һ���ֻ��Ǻ�תΪ����ɫ�����ԭ��
 ��
��
��5����ͬѧΪ�˱���M�缫������ʴ�������Խ�N�缫������Ϊ��д��ѧʽ�� ��Ϊ��֤�÷���������Ч�����������жԱ�ʵ�飺��ͨ��·2���Ӻֱ���M�缫������2�λ�ɫK3[Fe(CN)6]��Һ������û�и���N�缫�����ձ��е������� ��
 �� ��
�� �� ����ʾ�����ձ�����
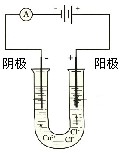
����ʾ�����ձ������������ƣ��á�
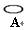 ����ʾ���������缫�����ֱ���M��N�����е绯ѧʵ��̽����
����ʾ���������缫�����ֱ���M��N�����е绯ѧʵ��̽������ͬѧ��װ���������Ӻ�ֱ����Դͨ�缸���ӣ�����M����Һ��dz��ɫ����һ��ʱ�䣬��Һ��û��������ֺ���ɫ��
��ͬѧ���õ������ͼ�ͬѧ�Ŀ���ȥ��ͬ�����Ӻ�ֱ����Դͨ�缸���ӣ�ȴ�ŵ�һ�ɴ̱ǵ���ζ������ֹͣͨ�硣
��ͬѧ��װ����������·�պϼ����Ӻ�ȴû�з�������������������ֺܿ�������������ƣ����ֵ����Ƶ�ָ�뷢����ƫת��
���������ͬѧ��ʵ����
 ��ش��������⣺
��ش��������⣺��1��M�缫�������ǣ�д��ѧʽ�� ��N�缫�������ǣ�д��ѧʽ�� ��
��2���������������ɶ�Ӧ����ͬѧ��װ��ͼ��

��3����Ҫ��д������ͬѧʵ��������漰�ķ�Ӧ����ʽ��
��ͬѧN�缫����ʽ
��ͬѧ�ܷ�Ӧ�����ӷ���ʽ
��ͬѧN�缫����ʽ
��4���û�ѧ����ʽ���ͼ�ͬѧʵ��ʱ�۲쵽M����Һ���ֻ��Ǻ�תΪ����ɫ�����ԭ��
 ��
����5����ͬѧΪ�˱���M�缫������ʴ�������Խ�N�缫������Ϊ��д��ѧʽ�� ��Ϊ��֤�÷���������Ч�����������жԱ�ʵ�飺��ͨ��·2���Ӻֱ���M�缫������2�λ�ɫK3[Fe(CN)6]��Һ������û�и���N�缫�����ձ��е������� ��
��1��Fe ��C��Pt
��2��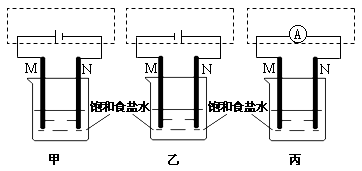
��3��2H++2e-===H2��
2Cl-+2H2O===Cl2��+H2��+2OH-
O2+2H2O+4e-
 ===4OH-
===4OH-
��4��4Fe(OH)2+O2+2H2O===4Fe(OH)3
��5��Zn�ȡ�������ɫ����
��2��

��3��2H++2e-===H2��
2Cl-+2H2O===Cl2��+H2��+2OH-
O2+2H2O+4e-

 ===4OH-
===4OH-��4��4Fe(OH)2+O2+2H2O===4Fe(OH)3
��5��Zn�ȡ�������ɫ����
��

��ϰ��ϵ�д�
�����Ŀ

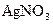 ��Һ�У�ͨ���⣬�����Һ��pH��6.0��Ϊ3.0ʱ(�������Һ���ǰ������ı仯���Բ���),��缫��Ӧ����������������
��Һ�У�ͨ���⣬�����Һ��pH��6.0��Ϊ3.0ʱ(�������Һ���ǰ������ı仯���Բ���),��缫��Ӧ����������������