��Ŀ����
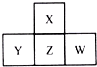 X��Y��Z��W���ֶ�����Ԫ�������ڱ��е����λ����ͼ��ʾ��������Ԫ�ص�����������֮��Ϊ24����ش��������⣮
X��Y��Z��W���ֶ�����Ԫ�������ڱ��е����λ����ͼ��ʾ��������Ԫ�ص�����������֮��Ϊ24����ش��������⣮��1��XԪ�������ڱ��е�λ��Ϊ
��2��Y��Z��W������������ˮ�����У��������������ʵĻ�ѧʽΪ
��3����һ����ѧ����ʽ˵��WԪ�صķǽ�����ǿ��ZԪ�صķǽ�����
��4��YԪ�ص�һ�ֵ��ʷ�������4��ԭ�ӹ��ɵģ����ֵ�����ȼ���ɵ��������������к���4��Yԭ�ӣ���31gY�ĸõ��ʳ������ڿ�������ȼʱ�ų�������Ϊa kJ��������ȼ��Ӧ���Ȼ�ѧ����ʽΪ
����������Ԫ�������ڱ��е�λ��֪��Xλ�ڵڶ����ڣ�Y��Z��Wλ�ڵ������ڣ�������Ԫ�ص�����������֮��Ϊ24����X��������������a��X��Z����ͬһ����Ԫ�أ�����Z����������Ҳ��a����Y��������������a-1��W��������������a+1��������Ԫ������������֮��=a-1+a+a+a+1=24��a=6������X��OԪ�أ�Y��PԪ�أ�Z��SԪ�أ�W��ClԪ�أ����Ԫ�ء����ʵ����ʷ������
����⣺����Ԫ�������ڱ��е�λ��֪��Xλ�ڵڶ����ڣ�Y��Z��Wλ�ڵ������ڣ�������Ԫ�ص�����������֮��Ϊ24����X��������������a��X��Z����ͬһ����Ԫ�أ�����Z����������Ҳ��a����Y��������������a-1��W��������������a+1��������Ԫ������������֮��=a-1+a+a+a+1=24��a=6������X��OԪ�أ�Y��PԪ�أ�Z��SԪ�أ�W��ClԪ�أ�
��1��X��OԪ�أ�Oԭ�Ӻ�����2�����Ӳ㣬������������6������Ԫ���У�ԭ�ӵĵ��Ӳ���������������������������������������������OԪ��λ�ڵڶ����ڵ�VIA�壬
�ʴ�Ϊ���ڶ����ڵ�VIA�壻
��2��ͬһ�����У�Ԫ�صķǽ���������ԭ���������������ǿ��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ��Y��Z��W������Ԫ���У��ǽ�������������PԪ�أ�����H3PO4������������
�ʴ�Ϊ��H3PO4��
��3��Ԫ�صķǽ�����Խǿ���䵥�ʵ�������Խǿ�������������ᷴӦ��������Ȼ��⣬H2S+Cl2=2HCl+S�����÷�Ӧ������������������������������������������Դ����÷�Ӧ��˵����Ԫ�صķǽ����Դ�����Ԫ�أ��ʴ�Ϊ��H2S+Cl2=2HCl+S����
��4�����Ļ�ѧʽΪP4������ȼ�������������������������к���4����ԭ�ӣ��ڸ��������У���Ԫ����+5�ۣ�����Ԫ�ػ��ϼ۵Ĵ�����Ϊ0֪����������Ļ�ѧʽΪP4O10��31gP4�����ʵ���=
=0.25mol��31gY�ĸõ��ʳ������ڿ�������ȼʱ�ų�������Ϊa kJ��1molP4ȼ�շų���������4akJ/mol������Ȼ�ѧ��Ӧ����ʽΪ��P4��s��+5O2��g��=P4O10��s����H=-4akJ/mol��
�ʴ�Ϊ��P4��s��+5O2��g��=P4O10��s����H=-4akJ/mol��
��1��X��OԪ�أ�Oԭ�Ӻ�����2�����Ӳ㣬������������6������Ԫ���У�ԭ�ӵĵ��Ӳ���������������������������������������������OԪ��λ�ڵڶ����ڵ�VIA�壬
�ʴ�Ϊ���ڶ����ڵ�VIA�壻
��2��ͬһ�����У�Ԫ�صķǽ���������ԭ���������������ǿ��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ��Y��Z��W������Ԫ���У��ǽ�������������PԪ�أ�����H3PO4������������
�ʴ�Ϊ��H3PO4��
��3��Ԫ�صķǽ�����Խǿ���䵥�ʵ�������Խǿ�������������ᷴӦ��������Ȼ��⣬H2S+Cl2=2HCl+S�����÷�Ӧ������������������������������������������Դ����÷�Ӧ��˵����Ԫ�صķǽ����Դ�����Ԫ�أ��ʴ�Ϊ��H2S+Cl2=2HCl+S����
��4�����Ļ�ѧʽΪP4������ȼ�������������������������к���4����ԭ�ӣ��ڸ��������У���Ԫ����+5�ۣ�����Ԫ�ػ��ϼ۵Ĵ�����Ϊ0֪����������Ļ�ѧʽΪP4O10��31gP4�����ʵ���=
| 31g |
| 124g/mol |
�ʴ�Ϊ��P4��s��+5O2��g��=P4O10��s����H=-4akJ/mol��
���������⿼����Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ�ã���ȷ�ƶ�Ԫ���ǽⱾ��ؼ������Ԫ������������������ѶȲ���

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ
��֪X��Y��Z��W���ֶ���������Ԫ�������ڱ��е����λ����ͼ��ʾ������˵����ȷ���ǣ�������
| X | Y |
| Z | W |
| A��W��ԭ������������Y��ԭ��������2�� |
| B��ZԪ�ص�ԭ�Ӱ뾶���ܱ�YԪ�ص�С |
| C��W����̬�⻯����ȶ���һ����Y��ǿ |
| D����Z���������Ϊ+m����X���������Ҳһ��Ϊ+m |
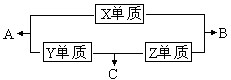 X��Y��Z��W���ֶ�����Ԫ�أ�����X��Y��Z��ԭ������֮��Ϊ16��X��Y��Z����Ԫ�صij��������ڳ��³�ѹ�¶�����ɫ���壬���ʵ��������¿��Է�������ͼ��ʾ�ı仯��
X��Y��Z��W���ֶ�����Ԫ�أ�����X��Y��Z��ԭ������֮��Ϊ16��X��Y��Z����Ԫ�صij��������ڳ��³�ѹ�¶�����ɫ���壬���ʵ��������¿��Է�������ͼ��ʾ�ı仯��
 ��X��Y��Z��W���ֶ�����Ԫ�ص�λ�ù�ϵ��ͼ��ʾ����֪WΪ�����а뾶��С��Ԫ�أ�ZΪ��������ԭ�Ӱ뾶��С��Ԫ�أ�
��X��Y��Z��W���ֶ�����Ԫ�ص�λ�ù�ϵ��ͼ��ʾ����֪WΪ�����а뾶��С��Ԫ�أ�ZΪ��������ԭ�Ӱ뾶��С��Ԫ�أ�
