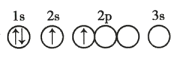
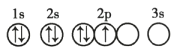
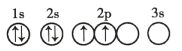
��Ŀ����
����Ŀ��������pC����Һ�������ʵ���Ũ�ȵĸ������������£�һ��Ũ�ȵ�ij��(H2A)��ˮ��Һ��pC(H2A)��pC(HA-)��pC(A2-)������ҺpH�ı仯������ͼ��ʾ��
����˵��һ����ȷ����(����)

A.pH=3ʱ��c(H2A)=c(A2-)>c(HA-)
B.c(H2A)��c(HA-)��c(A2-)�Ƕ�ֵ
C.HA-��ˮ��ƽ�ⳣ��Ϊ10-12.7
D.�κ�ʱ����Һ�ж����ڣ�c(H+)=c(OH-)��c(HA-)��2c(A2-)
���𰸡�C
��������
H2AH++HA-��HA-H++A2-��pH���Ӵٽ�����ƽ�������ƶ���������ͼ��֪����������HA-�����ʵ���Ũ�ȵĸ���������������H2A�����ʵ���Ũ�ȵĸ���������������A2-�����ʵ���Ũ�ȵĸ��������ɴ˷������
A����ͼ��֪pH=3ʱpC(H2A)=pC(A2-)> pC(HA-)������c(HA-)> c(H2A)=c(A2-)����A����
B�����������غ㣬n(H2A)��n(HA-)��n(A2-)Ϊ��ֵ������ҺpH�ı�ʱ��Һ��������ܷ����˸ı䣬��������Ũ��֮��һ���Ƕ�ֵ����B����
C��HA-��ˮ��ƽ�ⳣ������ʽΪ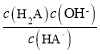 ����ͼ��֪��pH=1.3ʱc(HA-)=c(H2A)����Һ��c(H+)=10-1.3mol/L����c(OH-)=10-12.7mol/L������HA-��ˮ��ƽ�ⳣ��Ϊ10-12.7����C��ȷ��
����ͼ��֪��pH=1.3ʱc(HA-)=c(H2A)����Һ��c(H+)=10-1.3mol/L����c(OH-)=10-12.7mol/L������HA-��ˮ��ƽ�ⳣ��Ϊ10-12.7����C��ȷ��
D����ҺpH����Ĺ��̿��ܼ�����ij�ּ��Һ�����������������ӣ����ݵ���غ��֪c(H+)��c(OH-)��c(HA-)��2c(A2-)����ȣ���D����
�ʴ�ΪC��

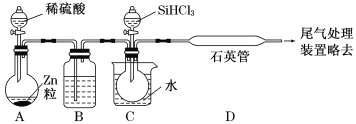
����Ŀ�����ж�һЩʵ����ʵ�����۽�����ȷ����
ѡ�� | ʵ����ʵ | ���۽��� |
A | HCl��������ˮ���ܵ��� | HClΪ���ӻ����� |
B | HBr������ǿ��HCl������ | Br�ķǽ����Ա�Clǿ |
C |
|
|
D | HF�ķе����HCl | F�ķǽ����Ա�Clǿ |
A.AB.BC.CD.D