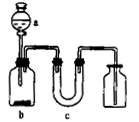
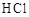��Ŀ����
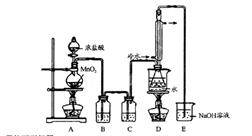
�⻯��(CaH2)������һ�ִ�����ϣ��ǵ�ɽ�˶�Ա���õ���Դ�ṩ�������ڼ���ʱ���뵪����������Ӧ���⻯����ˮ������Ӧ�����������ƺ��������⻯��ͨ��������������Ƽ�����ȡ����ͼ��ģ����ȡװ�á�

��1�����й����⻯�Ƶ�������ȷ���� (ѡ�����)��
a���⻯���������ӵİ뾶С��Li+�İ뾶
b���⻯�Ƶ�ʽ��С���廯�⣬���ǰ�ߵ��۵�С�ں���
c���⻯��Ҳ�������ᷴӦ��������
d��������������ԭ����ֻ���л�ԭ��
��2����ͼAװ�����Ʊ��������õ�����Һ���ѡ�� (ѡ�����)��
a��ϡ���� b��ϡ���� c��ϡ���� d��������
��3��װ��D����ֱ���ܵ������� ��
��4��Ϊ��ȷ�Ͻ���װ��C�������Ѿ��������B��C֮���ٽ�һװ�ã���װ���м�����Լ��� ������Cװ��ǰҪ��H2�鴿���鴿�IJ����� ��
��5����ͬѧ��ΪֻҪװ�ú����������淶�Ϳ����ų����� (ѡ�����)��
a��Ca3N2 b��CaO c��Ca(OH)2
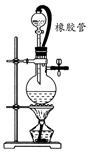
��6����ͬѧ����ͼװ�òⶨ�Ƶõ��⻯�ƵĴ��ȡ�����ȡ48g��Ʒ����������ˮ��Ӧ������ʱ��ע������������������Ϊ48.16 L(�ѻ���Ϊ��״��)�������ֻ�����������˷�Ӧ���������ͬѧ��ʵ�����ݼ����⻯�ƵĴ���(д���������) ��


��1�����й����⻯�Ƶ�������ȷ���� (ѡ�����)��
a���⻯���������ӵİ뾶С��Li+�İ뾶
b���⻯�Ƶ�ʽ��С���廯�⣬���ǰ�ߵ��۵�С�ں���
c���⻯��Ҳ�������ᷴӦ��������
d��������������ԭ����ֻ���л�ԭ��
��2����ͼAװ�����Ʊ��������õ�����Һ���ѡ�� (ѡ�����)��
a��ϡ���� b��ϡ���� c��ϡ���� d��������
��3��װ��D����ֱ���ܵ������� ��
��4��Ϊ��ȷ�Ͻ���װ��C�������Ѿ��������B��C֮���ٽ�һװ�ã���װ���м�����Լ��� ������Cװ��ǰҪ��H2�鴿���鴿�IJ����� ��
��5����ͬѧ��ΪֻҪװ�ú����������淶�Ϳ����ų����� (ѡ�����)��
a��Ca3N2 b��CaO c��Ca(OH)2
��6����ͬѧ����ͼװ�òⶨ�Ƶõ��⻯�ƵĴ��ȡ�����ȡ48g��Ʒ����������ˮ��Ӧ������ʱ��ע������������������Ϊ48.16 L(�ѻ���Ϊ��״��)�������ֻ�����������˷�Ӧ���������ͬѧ��ʵ�����ݼ����⻯�ƵĴ���(д���������) ��

��1��c
��2��b
��3��ƽ��ѹǿ����ֹ���浼�ܶ���
��4����ˮ����ͭ(������������)���ռ�һ�Թ����壬���ܿڿ����ƾ��ƻ��棬�����������ۡ�������
��5��a��b��c��
��6��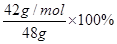 ��0.875
��0.875
��2��b
��3��ƽ��ѹǿ����ֹ���浼�ܶ���
��4����ˮ����ͭ(������������)���ռ�һ�Թ����壬���ܿڿ����ƾ��ƻ��棬�����������ۡ�������
��5��a��b��c��
��6��
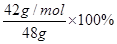 ��0.875
��0.875�����������1��a�У��⻯����H-��Li+�ĵ��ӹ�����ͬ��Li�ĺ˵�����뾶С��a˵������ȷ��b��⻯�������Ӿ��壬�廯���Ƿ��Ӿ��壬���ǰ�ߵ��۵���ں��ߣ�����ȷ��d�������Ca��Ӧ������������d����ȷ��
��2���Ʊ�H2ʱ��ʹ��ϡ���ᣬ�����HCl���壬Ҳ����Ca��Ӧ��ʹ��ϡ���ᣬ����Ϊ������������H2�����Բ��С�
��3��ϴ��ƿ�в���Һ���ڲ��ĵ����������ͨ����ƽ��ѹǿ����ֹ���浼�ܶ���(��ֱ�������岻��)��
��4������ˮ������һ��ʹ����ˮ����ͭ(����ˮ����δ��ȥ�������ɫ)������ˮ���ռ�һ�Թ����壬���ܿڿ����ƾ��ƻ��棬�����������ۡ�������
��5����ʼͨ��H2�������ų�װ���еĿ�����û��N2��O2��ˮ�������������Ca3N2��CaO��Ca(OH)2�����ʡ�
��6��42g��mol-1 n(CaH2)+40 g��mol-1n(Ca)=48
2n(CaH2)+n(Ca)=
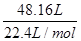 ��ã�n(CaH2)=1mol
��ã�n(CaH2)=1mol ���⻯�ƵĴ��ȣ�
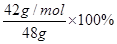 =87.5%��0.875��
=87.5%��0.875��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
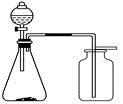
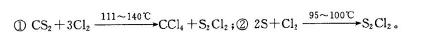 ��֪��S2Cl2����Ԫ����+1�ۣ�����ʽ��
��֪��S2Cl2����Ԫ����+1�ۣ�����ʽ��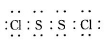 �������ȶ�����ˮ�������绯��Ӧ (һ������Ԫ�ؼ�̬���ߣ�һ���ֽ���)����Ӧ�漰�ļ������ʵ��۷е����£�
�������ȶ�����ˮ�������绯��Ӧ (һ������Ԫ�ؼ�̬���ߣ�һ���ֽ���)����Ӧ�漰�ļ������ʵ��۷е����£�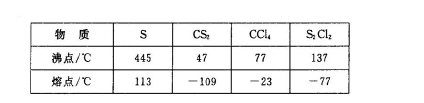
 H2
H2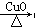 Cu
Cu Cu
Cu