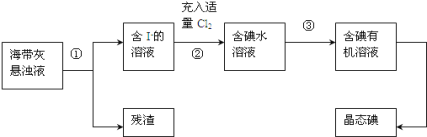
��Ŀ����
����Ŀ��ijѧϰС��̽�����Ļ�ԭ�Լ������������ʣ�����ͼװ�ý���ʵ��(ͼ�мг�װ������ȥ)����B�����������������������ȣ��ٹ����������֪����ˮ�Ȼ��ƿ����հ�����ˮ���ش��������⣺
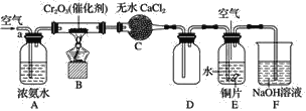
(1)B�з�����Ӧ�Ļ�ѧ����ʽ��______________________________��
(2)֤������������������������________________����Ӧ�ķ���ʽ��________________��
(3)E��ͭ������Ӧ�����ӷ���ʽ��________________________________��
(4)E�л���ͨ�������Ŀ����____________________________________��
���𰸡�4NH3��5O2![]() 4NO��6H2O D�й��ƿ���к���ɫ���� 2NO+O2===2NO2 3Cu��8H����2
4NO��6H2O D�й��ƿ���к���ɫ���� 2NO+O2===2NO2 3Cu��8H����2![]() ===3Cu2����2NO����4H2O ʹһ���������ת��Ϊ�������������������Ⱦ
===3Cu2����2NO����4H2O ʹһ���������ת��Ϊ�������������������Ⱦ
��������
�������������ɵ�NO�Ϳ����е�O2��Ӧ����NO2��NO2������ˮ����HNO3��Cu������ϡ��������NO����Ӧ��β��NO�Ϳ����Ļ��������NaOH��Һ���ա�
(1)B�з����ķ�Ӧ�ǰ��Ĵ�������Ӧ 4NH3��5O2![]() 4NO��6H2O��
4NO��6H2O��
(2)�����������IJ���һ������������������2NO��O2===2NO2��D�й��ƿ���к���ɫ���������
(3)��E�У����ɵĶ���������ˮ��Ӧ3NO2��H2O = 2HNO3��NO��ͭ�����ᷴӦ3Cu��8HNO3(ϡ) = 3Cu(NO3)2��2NO����4H2O�����ӷ���ʽΪ3Cu��8H����2![]() =3Cu2����2NO����4H2O��
=3Cu2����2NO����4H2O��
(4)Ϊʹһ���������ת��Ϊ�������������������Ⱦ����E��Ҫ����ͨ�������