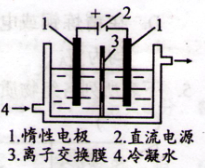
��Ŀ����
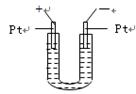
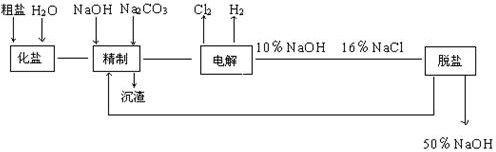
�ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ĺ�������ʾ��ͼ���£�
������ͼ�����������գ�
��1�����Դ���������ĵ缫��������ҺpHֵ��ѡ����䡢�����½��������Դ���������ĵ缫�� ��������ü��ϲ���ķ����� ��
��2��д����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ ��
��3�����������SO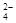 �����ϸߣ��������ӱ�ʽ����ȥSO
�����ϸߣ��������ӱ�ʽ����ȥSO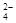 ���ñ��Լ���������ѡ��A.B.c����ѡ�۷֣�
���ñ��Լ���������ѡ��A.B.c����ѡ�۷֣�
A��Ba(OH)2 B��Ba(NO3)2 C��BaCl2
��4���жϱ��Լ��Ѿ������ķ����� ��
��5��Ϊ��Ч��ȥCa2+��Mg2+��SO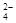 �������Լ��ĺ���˳��Ϊ��ѡ��a��b��c��ѡ�۷֣�
�������Լ��ĺ���˳��Ϊ��ѡ��a��b��c��ѡ�۷֣�
A���ȼ�NaOH��Һ�����Na2CO3��Һ���ټӱ��Լ�
B���ȼ�NaOH��Һ����ӱ��Լ����ټ�Na2CO3��Һ
C���ȼӱ��Լ������NaOH��Һ���ټ�Na2CO3��Һ
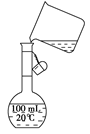
��6��Ϊ���龫�δ��ȣ�������150 mL0.2 mol/LNaCl�����Σ���Һ����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ����� ��

������ͼ�����������գ�
��1�����Դ���������ĵ缫��������ҺpHֵ��ѡ����䡢�����½��������Դ���������ĵ缫�� ��������ü��ϲ���ķ����� ��
��2��д����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ ��
��3�����������SO
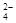 �����ϸߣ��������ӱ�ʽ����ȥSO
�����ϸߣ��������ӱ�ʽ����ȥSO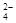 ���ñ��Լ���������ѡ��A.B.c����ѡ�۷֣�
���ñ��Լ���������ѡ��A.B.c����ѡ�۷֣� A��Ba(OH)2 B��Ba(NO3)2 C��BaCl2
��4���жϱ��Լ��Ѿ������ķ����� ��
��5��Ϊ��Ч��ȥCa2+��Mg2+��SO
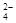 �������Լ��ĺ���˳��Ϊ��ѡ��a��b��c��ѡ�۷֣�
�������Լ��ĺ���˳��Ϊ��ѡ��a��b��c��ѡ�۷֣�A���ȼ�NaOH��Һ�����Na2CO3��Һ���ټӱ��Լ�
B���ȼ�NaOH��Һ����ӱ��Լ����ټ�Na2CO3��Һ
C���ȼӱ��Լ������NaOH��Һ���ټ�Na2CO3��Һ
��6��Ϊ���龫�δ��ȣ�������150 mL0.2 mol/LNaCl�����Σ���Һ����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ����� ��

��1������ ���� �����۵⻯����ֽ��ˮ��ʪ��ճ�ڲ�����һ��,����װ�д�������ļ���ƿ �������ɫ������ʹ��ֽ����ɫ,֤������Cl2��
��2����ⱥ��ʳ��ˮ�ķ�Ӧ��2NaCl+2H2O 2NaOH+Cl2��+H2��
2NaOH+Cl2��+H2��
��3��C
��4����ֹ�����ϲ���Һ�м����μ�BaCl2���粻�����������ѹ�����
��5��B.C��
��6��δ�ò��������� 100 mL������ƿ��������150 mL ��Һ
��2����ⱥ��ʳ��ˮ�ķ�Ӧ��2NaCl+2H2O
 2NaOH+Cl2��+H2��
2NaOH+Cl2��+H2����3��C
��4����ֹ�����ϲ���Һ�м����μ�BaCl2���粻�����������ѹ�����
��5��B.C��
��6��δ�ò��������� 100 mL������ƿ��������150 mL ��Һ
�����������1�����Դ��������Ϊ����������������NaOH�����ɼ����pH���ߣ�����������Ϊ���������������������۵⻯����ֽ��ˮ��ʪ��ճ�ڲ�����һ��,����װ�д�������ļ���ƿ �������ɫ������ʹ��ֽ����ɫ,֤������Cl2��
��2����ⱥ��ʳ��ˮ�ķ�Ӧ��2NaCl+2H2O
 2NaOH+Cl2��+H2��
2NaOH+Cl2��+H2����3�����ӱ��Լ���ȥSO42-��ע�ⲻ�������µ����ʣ�ѡBa��NO3��2������������������ӣ����Ըñ��Լ�����ѡ�á�ͬ��Ba(OH)2��������OH-���ӡ�
��4����ֹ�����ϲ���Һ�м����μ�BaCl2���粻�����������ѹ�����
SO42-��Ca2+��Mg2+�ȷֱ���BaCl2��Һ��Na2CO3��Һ��NaOH��Һ��Ӧ���ɳ���������ͨ�����˳�ȥ��Na2CO3��Һ�ܳ�ȥ������BaCl2��Һ�������ܳ�ȥ������Na2CO3��Һ��NaOH��Һ������Ӧ�ȼ�BaCl2��Һ�ټ�Na2CO3��Һ�����������ᡣ�ʴ�Ϊ:B.C��
��6��δ�ò�����������100 mL������ƿ��������150 mL ��Һ��

��ϰ��ϵ�д�
�����Ŀ
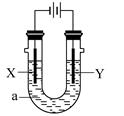
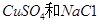 �Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ����
�Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ����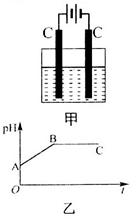
 ������A����Һ��pHֵС��B��
������A����Һ��pHֵС��B��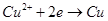
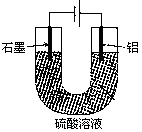
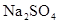 ��Һ��ֱͨ���磬һ��ʱ���U�ι��ڻ��γ���ɫ���ʺ硱����������������ɫ�Ĵ����ǣ��� ��
��Һ��ֱͨ���磬һ��ʱ���U�ι��ڻ��γ���ɫ���ʺ硱����������������ɫ�Ĵ����ǣ��� ��