��Ŀ����
����Ŀ����ˮ������������ѧʵ�����г����Ļ�ѧ�Լ��������ڹ�ũҵ������Ҳ���й㷺Ӧ�á�ij�о���ѧϰС��Ϊ�ⶨ��ˮ��Ũ�ȣ����ð�ˮ��Ϊ�ᴿ����ʱ���Լ��������������������ʵ�����£�
�������ϣ�
�ټ��ȵı�ɫ��Χ��pH��3.1��ɫ��pH��3.1��4.4��ɫ�� pH��4.4��ɫ
�ڷ�̪�ı�ɫ��Χ��pH��8.2��ɫ ��pH��8.2��10.0�ۺ�ɫ��pH��10.0��ɫ
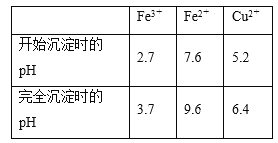
����֪��Fe3����Fe2����Cu2��ת��Ϊ��������ʱ��Ӧ��pH���±���
ʵ��һ���궨��ˮ��Ũ��
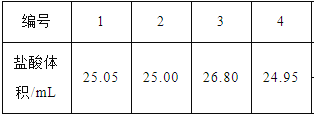
ȡ25.00mLԼΪ0.10 mol��L��1��ˮ����ƿ�У���0.1000 mol��L��1������еζ���ʵ�������������±���ʾ��
��1���ζ�����ˮ������ӷ���ʽΪ____________________________________���ɴ˿���֪ѡ��ĵζ�ָʾ��ӦΪ__________________��������������������̪����
��2���ð�ˮ��ȷŨ��Ϊ____________________mol��L��1������ȷ��С�������λ��
��3�����3����Һ������Ũ���ɴ�С��˳��Ϊ__________________________________��
ʵ��� �ᴿ��������
ijѧϰС��ͬѧ��Ӻ�FeSO4��Fe2(SO4)3���ʵ�CuSO4��Һ���ᴿ����������Ҫʵ�鲽�����£�
��һ�� �����Һ�м���3% H2O2��Һ��ַ�Ӧ���ټ���ϡ��ˮ������ҺpH�����ˡ�
�ڶ��� ����Һ�м���ϡ���������ҺpH��1��2���ᴿ������
��4������3% H2O2��Һ��������___________________________��
��5����ϡ��ˮ����pHӦ������Χ___________________________��
��6���������ʿ��������ϡ��ˮ����___________________________��������ţ�
A��NaOH B��Cu(OH)2 C��CuO D��NaHCO3
���𰸡�NH4����H2O![]() NH3��H2O��H�� ���� 0.1000 [Cl��]>[NH4��]>[H��]>[OH��] ��[Cl��] [NH4��] [H��] [OH��] ����Һ�е�Fe2������ΪFe3�������Һ�е�FeSO4����ΪFe2��SO4��3 5.2>pH��3.7 ��3.7��pH<5.2 ��[3.7��5.2�� BC
NH3��H2O��H�� ���� 0.1000 [Cl��]>[NH4��]>[H��]>[OH��] ��[Cl��] [NH4��] [H��] [OH��] ����Һ�е�Fe2������ΪFe3�������Һ�е�FeSO4����ΪFe2��SO4��3 5.2>pH��3.7 ��3.7��pH<5.2 ��[3.7��5.2�� BC
��������
(1).������ζ���ˮ���ζ������Ȼ����ǿ�������Σ���Һ�����ԣ�Ҫѡ�������Է�Χ�ڱ�ɫ��ָʾ����
��2������c(NH3��H2O)V(NH3��H2O)=c(HCl)V(HCl)���㰱ˮ��Ũ�ȣ�
��3�����3��ʵ�������������Һ�к����Ȼ�李����
��4����ȥ��Һ�е���Ԫ�أ���������������ͭ��������Ҫ��Fe2������ΪFe3����
��5������Fe3����ȫ������Cu2�����ܳ������жϵ���pH�ķ�Χ��
��6�����������ϡ��ˮ�����ʣ���Ҫ���ϵ������ǣ���ʹ��ҺPH���߶������������ʡ�
(1). �ζ������Ȼ����ǿ�������Σ�笠�����ˮ�⣬��Һ�����ԣ�ˮ������ӷ���ʽ��NH4����H2O![]() NH3��H2O��H�����Ȼ����Һ�����ԣ�Ҫѡ�������Է�Χ�ڱ�ɫ��ָʾ������ѡ���ȣ�
NH3��H2O��H�����Ȼ����Һ�����ԣ�Ҫѡ�������Է�Χ�ڱ�ɫ��ָʾ������ѡ���ȣ�
��2��������ʵ������ƫ����������Χ��ȥ������1��2��4�������ݣ�ƽ����������������25.00mL��c(NH3��H2O)V(NH3��H2O)=c(HCl)V(HCl)��c(NH3��H2O)= 0.1000 mol��L��1��0.025L��0.025L=0.1000 mol��L��1��
��3�����3��ʵ�������������Һ�к����Ȼ�李����ᣬ��������Ũ��[Cl��]>[NH4��]>[H��]>[OH��]��
��4����ȥ��Һ�е���Ԫ�أ���������������ͭ��������Ҫ��Fe2������ΪFe3�������������Һ�м���3% H2O2��Һ�������ǰ���Һ�е�Fe2������ΪFe3����
��5��ʹFe3����ȫ������Cu2�����ܳ��������Ե���pH�ķ�Χ��3.7��pH<5.2��
��6��Cu(OH)2��CuO��ʹ��ҺPH���߶������������ʣ����������ϡ��ˮ��NaOH��NaHCO3���������ʣ����ܴ���ϡ��ˮ����ѡBC��
