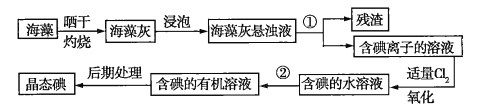
��Ŀ����
����Ŀ����1������A����Է���������65-75֮�䣬1molA��ȫȼ������7.5mol��������A�Ľṹ��_______�֡�
��2���л���B������ˮ���ᣬ��ṹ��ʽΪ ����B��һ����ҺC(����)��Ӧ�ɵõ�һ������(�仯ѧʽΪC7H5O3Na)����C�Ļ�ѧʽΪ_______��ˮ�����ͬ���칹���У����ڷ��࣬�����������࣬Ҳ������������Ļ������бض�����_______(��д�����ǻ�����Ĺ���������)
����B��һ����ҺC(����)��Ӧ�ɵõ�һ������(�仯ѧʽΪC7H5O3Na)����C�Ļ�ѧʽΪ_______��ˮ�����ͬ���칹���У����ڷ��࣬�����������࣬Ҳ������������Ļ������бض�����_______(��д�����ǻ�����Ĺ���������)
��3��������![]() ��Ϳ���������ڱ��棬�������������̻�������������(������Ӧ���ɸ߷��ӻ�����)���ɴ���ͨ���˿ڵķ���ߣ��ø߷��ӻ�����Ľṹ��ʽΪ_______��
��Ϳ���������ڱ��棬�������������̻�������������(������Ӧ���ɸ߷��ӻ�����)���ɴ���ͨ���˿ڵķ���ߣ��ø߷��ӻ�����Ľṹ��ʽΪ_______��
��4��������Ƴ�һ�����;������� ����ȡ������ϩ����������������ɫ��Ⱦ�����������������ĵ���ṹ��ʽΪ_______��
����ȡ������ϩ����������������ɫ��Ⱦ�����������������ĵ���ṹ��ʽΪ_______��
���𰸡���1��5��2��NaHCO3��ȩ����3�� ��4��
��4��![]()
��������
�����������1����A�ķ���ʽΪCxHy��l mol A��ȫȼ������7mol��������x+![]() =7.5����65��12x+y��75��x��yΪ������������ã�x=5��y=10����AΪC5H10������A�Ľṹ�У�������5��̼����2�֣�������4��̼����3�֣���5�����ʴ�Ϊ��5��
=7.5����65��12x+y��75��x��yΪ������������ã�x=5��y=10����AΪC5H10������A�Ľṹ�У�������5��̼����2�֣�������4��̼����3�֣���5�����ʴ�Ϊ��5��
��2��A�к���-OH��-COOH���õ�һ�����Σ��仯ѧʽΪC7H5O3Na������ֻ��-COOH������Ӧ����CΪ̼��������Һ��ˮ�����ͬ���칹���У����ڷ��࣬�����������࣬Ҳ������������Ļ�����һ�����з�-OH��-CHO���ʴ�Ϊ��NaHCO3��ȩ��
��3��������![]() �е�̼̼˫�������Ӿ۷�Ӧ�����ɵ��߷��ӻ�����Ľṹ��ʽΪ
�е�̼̼˫�������Ӿ۷�Ӧ�����ɵ��߷��ӻ�����Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ��
��4�������������� �����ĵ���ṹ��ʽΪ
�����ĵ���ṹ��ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�����Ŀ����H2O2��KI��ϴ�ྫ�����������������ʵ�飨��ʱ���ڲ���������ĭ����ijͬѧ�����������϶Ը�ʵ�����̽����
(1)����1��KI�ڸ÷�Ӧ�е����ã�H2O2 + I![]() = H2O + IO
= H2O + IO![]() ��H2O2 + IO
��H2O2 + IO![]() = H2O + O2��+ I
= H2O + O2��+ I![]() ���ܷ�Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
���ܷ�Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
(2)����2��H2O2�ֽⷴӦ�����������仯��ͼ��ʾ�����Т���KI���룬����KI���롣�����ж���ȷ����______������ĸ����
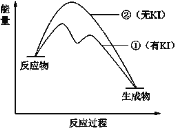
a������KI��ı��˷�Ӧ��·��
b������KI��ı����ܷ�Ӧ�������仯
c��H2O2 + I![]() = H2O + IO
= H2O + IO![]() �Ƿ��ȷ�Ӧ
�Ƿ��ȷ�Ӧ
(3)ʵ���з��֣�H2O2��KI��Һ��Ϻ����������ݣ���Һ��ɫ��ơ��ټ���CCl4�������ã��������Լ��١�
����3��I2Ҳ�ɴ�H2O2�ķֽⷴӦ��
�� ��CCl4�������úɹ۲쵽___________________________________��˵����I2���ɡ�
�� �������Լ��ٵ�ԭ������ǣ�
��. H2O2Ũ�Ƚ��ͣ�
��._________________________________________��
���¶���ʵ��˵����������Ҫԭ����H2O2��Һ�м���KI��Һ������Һ��ƺֳ����ȷ���A��B���Թ��С�A�Թܼ���CCl4��B�Թܲ���CCl4���ֱ������á��۲쵽��������________________________��
(4)����4��I![]() + I
+ I![]()
![]() I
I![]() K= 640��
K= 640��
Ϊ��̽����ϵ�к������Ĵ�����ʽ������ʵ�飺��20 mLһ��Ũ�ȵ�H2O2��Һ�м���10 mL 0.10 mol��L-1 KI��Һ����ƽ��������Ũ�����£�
�� | I | I | I |
Ũ��/ (mol��L-1) | 2.5��10-3 | a | 4.0��10-3 |
�� a =____________________��
�� ��ƽ����ϵ�г��˺���I![]() ��I
��I![]() ��I
��I![]() �⣬һ��������������������������_____________________��
�⣬һ��������������������������_____________________��