��Ŀ����
�������ѧ��ѧ֪ʶ�ش��������⣺
��1����Ũ�Ⱦ�Ϊ0.1mol/L ��CH3COONH4����NH4HSO4����NH3?H2O���ܣ�NH4��2SO4��Һ�У�NH4+Ũ���ɴ�С��˳��Ϊ ������ţ���
��2���Ȼ�����Һ���ɣ����յõ��Ĺ��������� ���û�ѧ����ʽ˵����ԭ��
��3��ij�¶�ʱ����ˮ��pH=6����2.3g�����Ʒ��������ˮ�У���ַ�Ӧ���ټӸ�����ˮϡ�͵�1L���ָ���ԭ�¶�ʱ������Һ��pH= ��
��1����Ũ�Ⱦ�Ϊ0.1mol/L ��CH3COONH4����NH4HSO4����NH3?H2O���ܣ�NH4��2SO4��Һ�У�NH4+Ũ���ɴ�С��˳��Ϊ
��2���Ȼ�����Һ���ɣ����յõ��Ĺ���������
��3��ij�¶�ʱ����ˮ��pH=6����2.3g�����Ʒ��������ˮ�У���ַ�Ӧ���ټӸ�����ˮϡ�͵�1L���ָ���ԭ�¶�ʱ������Һ��pH=
���㣺����Ũ�ȴ�С�ıȽ�,pH�ļ���,����ˮ���Ӧ��
ר�⣺����ƽ������Һ��pHר��,�����ˮ��ר��
��������1��һˮ�ϰ�Ϊ������ʣ���Һ��c��NH4+����С����ͬŨ�ȵ������Һ�У�NH4+ϵ��Խ��c��NH4+��Խ����ͬϵ������ͬŨ�ȵ������Һ�У�NH4+ˮ��̶�Խ������Һ��c��NH4+��ԽС��H+����笠�����ˮ�⡢CH3COO-�ٽ�NH4+ˮ�⣻
��2���Ȼ�����ˮ�����������������Ȼ�����лӷ��ԣ����ȴٽ��Ȼ���ӷ���������Һʱ�õ�Al��OH��3���壬����Al��OH��3����õ�Al2O3��
��3��ij�¶�ʱ����ˮ��pH=6����ˮ�����ӻ�����K=10-12��2.3gNa�����ʵ���Ϊ0.1mol������ԭ���غ��n��NaOH��=n��Na��=0.1mol����C��NaOH��=
=0.1mol/L���ٽ�����ӻ�����������Һ��������Ũ�ȣ��Ӷ�ȷ����ҺpH��
��2���Ȼ�����ˮ�����������������Ȼ�����лӷ��ԣ����ȴٽ��Ȼ���ӷ���������Һʱ�õ�Al��OH��3���壬����Al��OH��3����õ�Al2O3��
��3��ij�¶�ʱ����ˮ��pH=6����ˮ�����ӻ�����K=10-12��2.3gNa�����ʵ���Ϊ0.1mol������ԭ���غ��n��NaOH��=n��Na��=0.1mol����C��NaOH��=
| 0.1mol |
| 1L |
���
�⣺��1��һˮ�ϰ�Ϊ������ʣ���Һ��c��NH4+����С����ͬŨ�ȵ������Һ�У�NH4+ϵ��Խ��c��NH4+��Խ����ͬϵ������ͬŨ�ȵ������Һ�У�NH4+ˮ��̶�Խ������Һ��c��NH4+��ԽС��H+����笠�����ˮ�⡢CH3COO-�ٽ�NH4+ˮ�⣬������ͬŨ�ȵ��⼸����Һ��c��NH4+���ɴ�С��˳��Ϊ�ܣ��ڣ��٣��ۣ��ʴ�Ϊ���ܣ��ڣ��٣��ۣ�
��2���Ȼ�����ˮ�����������������Ȼ�����лӷ��ԣ����ȴٽ��Ȼ���ӷ����Ӷ��ٽ��Ȼ���ˮ�⣬��������Һʱ�õ�Al��OH��3���壬����Al��OH��3����ʱ�ֽ�õ�Al2O3����Ӧ����ʽΪ���ʴ�Ϊ��Al2O3��Al3++3H2O?Al��OH��3+3 H+��2Al��OH��3
Al2O3+H2O��
��3��ij�¶�ʱ����ˮ��pH=6����ˮ�����ӻ�����K=10-12��2.3gNa�����ʵ���Ϊ0.1mol������ԭ���غ��n��NaOH��=n��Na��=0.1mol����C��NaOH��=
=0.1mol/L����Һ��c��H+��=10-11 mol/L����pHΪ11��
�ʴ�Ϊ��11��
��2���Ȼ�����ˮ�����������������Ȼ�����лӷ��ԣ����ȴٽ��Ȼ���ӷ����Ӷ��ٽ��Ȼ���ˮ�⣬��������Һʱ�õ�Al��OH��3���壬����Al��OH��3����ʱ�ֽ�õ�Al2O3����Ӧ����ʽΪ���ʴ�Ϊ��Al2O3��Al3++3H2O?Al��OH��3+3 H+��2Al��OH��3
| ||
��3��ij�¶�ʱ����ˮ��pH=6����ˮ�����ӻ�����K=10-12��2.3gNa�����ʵ���Ϊ0.1mol������ԭ���غ��n��NaOH��=n��Na��=0.1mol����C��NaOH��=
| 0.1mol |
| 1L |
�ʴ�Ϊ��11��
���������⿼��������Ũ�ȴ�С�Ƚϡ�����ˮ�⡢pH�ļ����֪ʶ�㣬��������ˮ��̶ȴ�С��������ʵĵ���ȷ������Ũ�ȴ�С���ٽ�����ʵ����ʷ������ע�⣨3����ˮ�����ӻ������ļ��㷽����Ϊ�״��㣮

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A���ڡ�ʳ�����Ậ���IJⶨ��ʵ���У�ѡ���̪��ҺΪָʾ��������Һ����ɫ��Ϊ�ۺ�ɫ���ڰ�����ڲ���ɫ�����ﵽ�ζ��յ� |
| B���ڡ�������ijЩ��Ҫ�ɷֵļ��顱ʵ���У�������������ˮ���衢���÷����ij�����Һ�м�������������Cu��OH��2�����������ɫ����ʱ��˵�������к��и��� |
| C���ڡ���˾ƥ�ֵĺϳɡ�ʵ���У��Ѵ������������ľ�����г��ˣ��þƾ�ϴ�Ӿ���1��2�Σ�Ȼ����ˣ�������ת�Ƶ��������ϣ���������������������� |
| D�����������������Ȼ�������ʱ�������������ƾ������ѵĻ�������ⶾ�����������ж��ݿ�ʱҪ���Ͻ����˹����� |
��������Ϊ1.15g����״��ͬ����С������Ʒֱ�Ͷ������ˮ��������ˮ�Ҵ��У���ͬ�����²�������������ͷ�Ӧʱ��Ĺ�ϵͼʾ��ȷ���ǣ�������
A�� |
B�� |
C��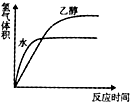 |
D�� |
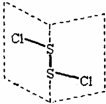 ���Ȼ�����S2Cl2���ǹ㷺������ҵ����������ӽṹ��ͼ��ʾ����ˮ��ˮ�⣬��������ʹƷ����ɫ�����壮S2Br2��S2Cl2�ṹ���ƣ�����˵��������ǣ�������
���Ȼ�����S2Cl2���ǹ㷺������ҵ����������ӽṹ��ͼ��ʾ����ˮ��ˮ�⣬��������ʹƷ����ɫ�����壮S2Br2��S2Cl2�ṹ���ƣ�����˵��������ǣ�������| A��S2Cl2����ԭ�ӹ���ӻ�����Ϊsp3�ӻ� |
| B��S2Cl2Ϊ���м��Լ��ͷǼ��Լ��ķǼ��Է��� |
| C���۷е㣺S2Br2��S2Cl2 |
| D��S2Cl2��H2O��Ӧ�Ļ�ѧ����ʽ����Ϊ��2S2Cl2+2H2O�TSO2��+3S��+4HCl |
ȡ100mL 0.3mol/L��300mL 0.25mol/L������ע��500mL����ƿ�У���ˮϡ�����̶��ߣ��û����Һ��H+�����ʵ���Ũ���ǣ�������mol/L��
| A��0.21 | B��0.42 |
| C��0.56 | D��0.26 |
����Ԫ������������ǣ�������
| A����������� | B������������ |
| C���˵���� | D�������� |