��Ŀ����
����Ŀ������β���dz��е���Ҫ������Ⱦ��о���������β����Ϊ������������Ҫ����
��1��������ȼ������ʱ������Ӧ��N2(g)+O2(g)![]() 2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T��ʱ����5L�ܱ������г���6.5molN2��7.5molO2����5minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5mol��
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T��ʱ����5L�ܱ������г���6.5molN2��7.5molO2����5minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5mol��
��5min�ڸ÷�Ӧ��ƽ��������(NO)=___����T��ʱ���÷�Ӧ��ƽ�ⳣ��K=___��
�ڷ�Ӧ��ʼ���ﵽƽ��Ĺ����У����������и�����仯����___������ţ���
a.���������ܶ�
b.��������ѹǿ
c.����Ӧ����
d.��λʱ���ڣ�N2��NO��������֮��
��2��H2��CO���Դ���ԭNO�Դﵽ������Ⱦ��Ŀ�ġ�
��֪��N2(g)+O2(g)=2NO(g) ��H=+180.5kJ��mol-1
2H2(g)+O2(g)=2H2O(l) ��H=-571.6kJ��mol-1
��H2(g)��NO(g)��Ӧ����N2(g)��H2O(l)���Ȼ�ѧ����ʽ��___��
���𰸡�0.2mol��L-1��min-1 1.25 cd 2H2(g)+2NO(g)=N2(g)+2H2O(l) ��H=��752.1kJ��mol-1
��������
(1)����������ʽ�г���������ݣ����뻯ѧ��Ӧ���ʺͻ�ѧƽ�ⳣ������ʽ���㣻
��a�������������������䣬������������䣻
b����������ܵ����ʵ������䣬����������䣻
c���淴Ӧ���У���Ӧ��Ũ�Ƚ��ͣ�����Ӧ�������ͣ�
d���淴Ӧ���У���Ӧ��Ũ�Ƚ��ͣ�����Ӧ�������ͣ��������Ũ�������淴Ӧ�������ʵ�λʱ���ڣ�N2����������С��NO�����������ݴ˷����жϣ�
(2)���ݸ�˹���ɷ������㡣
(1)��T��ʱ���� 5L�ܱ������г���6.5molN2��7.5molO2����5minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5mol���������������ݿ�֪��

���(NO)=![]() =0.2mol/(Lmin)��K=
=0.2mol/(Lmin)��K=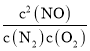 =
=![]() =1.25��
=1.25��
�ʴ�Ϊ��0.2mol/(Lmin)��1.25��
��a�������������������䣬������������䣬���������ܶȲ��䣬��a��ѡ��
b����������ܵ����ʵ������䣬����������䣬��������ѹǿ���䣬��b��ѡ��
c���淴Ӧ���У���Ӧ��Ũ�Ƚ��ͣ�����Ӧ�������ͣ���cѡ��
d���淴Ӧ���У���Ӧ��Ũ�Ƚ��ͣ�����Ӧ�������ͣ��������Ũ�������淴Ӧ�������ʵ�λʱ���ڣ�N2����������С��NO������������λʱ���ڣ�N2��NO��������֮�ȼ�С����dѡ��
�ʴ�Ϊ��cd��
(2)����֪����N2(g)+O2(g)=2NO(g) ��H=+180.5kJ/mol����2H2(g)+O2(g)=2H2O(l) ��H=-571.6 kJ/mol�����ݸ�˹���ɿ�֪��-�ٵã�2H2(g)+2NO(g)=N2(g)+2H2O(l) ��H=(-571.6kJ/mol) - 180.5kJ/mol �T -752.1kJ/mol��
�ʴ�Ϊ��2H2(g)+2NO(g)=N2(g)+2H2O(l) ��H=-752.1kJ/mol��

 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�