��Ŀ����
����Ŀ��Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ�����������Ч���ƿ����е������̼����������������Ե���Ϊ��Ҫ��
(1)�������������ڰ��մ�ת�������ɽ�����β������Ҫ��Ⱦ��ת��Ϊ���Ĵ���ѭ�����ʡ�
��֪����N2(g)��O2(g)��2NO(g) ��H��+180.5kJ��mol-1
��C��CO��ȼ����(��H)�ֱ�Ϊ-393.5kJ��mol-1��-283kJ��mol-1
��2NO(g)��2CO(g)��N2(g)��2CO2(g))��H��_______kJ��mol-1
(2)��0.20molNO��0.10molCO����һ���ݻ�Ϊ1L���ܱ������У���Ӧ����������Ũ�ȱ仯��ͼ��ʾ��
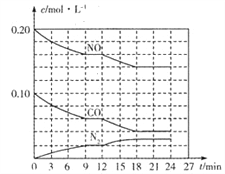
��CO��0-9min�ڵ�ƽ����Ӧ����v(CO)=________mol��L-1��min-1 (������λ��Ч���֣�����12 minʱ�ı�ķ�Ӧ��������Ϊ________��
A.�����¶� B.����NO C.�Ӵ��� D.�����¶�
�ڸ÷�Ӧ�ڵ�18 minʱ�ﵽƽ��״̬��CO2���������Ϊ________��������λ��Ч���֣�����ѧƽ�ⳣ��K=________��������λ��Ч���֣���
(3)ͨ���˹���������ܽ�ˮ��ȼú������CO2ת����HCOOH��O2����֪������0.1mol��L-2��
HCOONa��ҺpH=10����HCOOH�ĵ��볣��Ka=______________��
���𰸡�-746.54.4��l0-3D22.2%3.41.0��l0-7
����������1��N2��g��+O2��g���T2NO��g������H=+180.5kJ/mo1��
CO��g��+![]() O2��g���TCO2��g������H=-283kJ/mo1��
O2��g���TCO2��g������H=-283kJ/mo1��
C��s��+O2��g���TCO2��g������H=-393.5kJ/mo1��
���ݸ�˹���ɿɵã�����2-����2NO��g��+2CO��g��=N2��g��+2CO2��g����H=(-283kJ/mo1)��2-(+180.5kJ/mo1)=-746.5kJmol-1��
��2��v��CO��=![]() =
=![]() mol/��L��min��=4.4��10-3mol/��L��min����12minʱ�ı�����˲������Ũ�Ȳ��䣬������Ũ������NO��COŨ�ȼ�С��ƽ�������ƶ�������ӦΪ���ȷ�Ӧ��Ӧ�ǽ����¶ȣ��ʴ�ΪD��
mol/��L��min��=4.4��10-3mol/��L��min����12minʱ�ı�����˲������Ũ�Ȳ��䣬������Ũ������NO��COŨ�ȼ�С��ƽ�������ƶ�������ӦΪ���ȷ�Ӧ��Ӧ�ǽ����¶ȣ��ʴ�ΪD��
��3���÷�Ӧ�ڵ�24minʱ�ﵽƽ��״̬��ƽ��Ũ��c��N2��=0.03mol/L��c��NO��=0.14mol/L��c��CO��=0.04mol/L�����ݻ�ѧƽ������ʽ��ʽ����
2NO��g��+2CO��g���TN2��g��+2CO2��g��
��ʼ����mol/L�� 0.20.1 0 0
�仯����mol/L�� 0.06 0.060.03 0.06
ƽ������mol/L�� 0.14 0.04 0.03 0.06
CO2���������=![]() ��100%=22.2% ��ƽ�ⳣ��K=
��100%=22.2% ��ƽ�ⳣ��K=![]() =3.4��
=3.4��
��3�������£�0.1mol/L��HCOONa��ҺpHΪ10����Һ�д���HCOO-ˮ��HCOO-+H2O![]() HCOOH+OH-����Kh=
HCOOH+OH-����Kh=![]() =10-7����HCOOH�ĵ��볣��Ka=
=10-7����HCOOH�ĵ��볣��Ka=![]() =
=![]() =1��10-7��
=1��10-7��

����Ŀ��T��ʱ����A��B��0.32 mol��������ܱ������У�������Ӧ��A(g)��B(g)![]() 2C(g) ��H=��a kJ��mol��1 (a>0)����Ӧ�����вⶨ���������±�������˵����ȷ���ǣ� ��
2C(g) ��H=��a kJ��mol��1 (a>0)����Ӧ�����вⶨ���������±�������˵����ȷ���ǣ� ��
t/min | 0 | 2 | 4 | 7 | 9 |
n(B)/mol | 0.32 | 0.24 | 0.22 | 0.20 | 0.20 |
A������ʼʱ�������г���0.64 mol C�����ƽ��ʱ���յ�����Ϊ0.12a kJ
B�����£� ���ѹ�����������B��Ũ�Ⱥ��������������
C������ʼʱ�������г��� 0.64 mol A �� 0.64 mol B�� ���ƽ��ʱ n(C)<0.40 mol
D�����¡����ݣ���ƽ����ϵ���ٳ���0.32 mol A���ٴ�ƽ��ʱ��B��ת��������