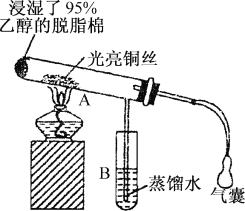
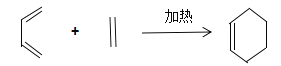��Ŀ����
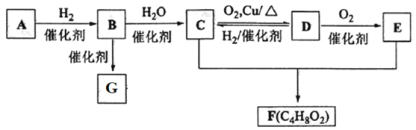
����Ŀ����֪��̬��A�ڱ�״���µ��ܶ���1.16 g��L1��B�IJ���������������һ������ʯ�ͻ�����չˮƽ��G��һ�ָ߷��ӻ�������� A��B��C��D��E��F��G �������¹�ϵ��
��ش�
(1)D�еĹ�����������_______________�� B�ĽṹʽΪ_________��
(2)д�� C+E��F��Ӧ�Ļ�ѧ����ʽ__________��
(3)д�� C��D��Ӧ�Ļ�ѧ����ʽΪ__________��
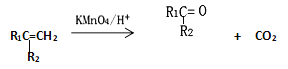
(4)��֪������ A ��һ�������¿ɺϳɲ���ʹ���� KMnO4 ��Һ��ɫ���л��д���úϳɷ�Ӧ�Ļ�ѧ����ʽ___________����Ӧ����Ϊ___________��
(5)�����й�������ȷ����_____________��
a. A��B��C��D��E��F��G��Ϊ�ǵ����
b. A������ԭ�Ӳ����ܴ���ͬһƽ����
c. ����ʱ��D ��������������ͭ����Һ��Ӧ����ש��ɫ����
d.75%(�������)�� C ˮ��Һ������ҽ������
e. ���̶���С���ƿ�Ͷ�� C �У��ƿ鸡��Һ���ϣ����д������ݲ���
���𰸡�ȩ�� ![]() CH3CH2OH+ CH3COOH
CH3CH2OH+ CH3COOH![]() CH3COOCH2CH3+H2O 2CH3CH2OH��O2
CH3COOCH2CH3+H2O 2CH3CH2OH��O2![]() 2CH3CHO��2H2O 3CH��CH
2CH3CHO��2H2O 3CH��CH![]()
![]() �ӳɷ�Ӧ cd
�ӳɷ�Ӧ cd
��������
��̬��A�ڱ�״���µ��ܶ���1.16 g��L1��M =22.4��= 22.4L��mol1��1.16 g��L1= 26g/mol��B�IJ���������������һ������ʯ�ͻ�����չˮƽ��˵��BΪCH2=CH2����AΪHC��CH��CΪCH3CH2OH��C(CH3CH2OH)��������ΪD(CH3CHO)��D(CH3CHO)��������ΪE(CH3COOH)��������Ҵ�������Ӧ����F(CH3COOCH2CH3)��G��һ�ָ߷��ӻ������GΪ����ϩ��
(1)DΪCH3CHO��������������ȩ����B����ϩ����ṹʽΪ![]() ���ʴ�Ϊ��ȩ����
���ʴ�Ϊ��ȩ����![]() ��
��
(2)C+E��F��������Ҵ�����������Ӧ���䷴Ӧ�Ļ�ѧ����ʽCH3CH2OH+ CH3COOH![]() CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3CH2OH+ CH3COOH
CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3CH2OH+ CH3COOH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
(3)C��D���Ҵ���������Ϊ��ȩ���䷴Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH��O2![]() 2CH3CHO��2H2O���ʴ�Ϊ��2CH3CH2OH��O2
2CH3CHO��2H2O���ʴ�Ϊ��2CH3CH2OH��O2![]() 2CH3CHO��2H2O��
2CH3CHO��2H2O��
(4)��֪������A��һ�������¿ɺϳɲ���ʹ����KMnO4��Һ��ɫ���л�����ݺϳɷ�Ӧ���ص��ԭ���غ�õ����л���Ϊ�����úϳɷ�Ӧ�Ļ�ѧ����ʽ3CH��CH![]()
![]() ����Ӧ����Ϊ�ӳɷ�Ӧ���ʴ�Ϊ��3CH��CH
����Ӧ����Ϊ�ӳɷ�Ӧ���ʴ�Ϊ��3CH��CH![]()
![]() ���ӳɷ�Ӧ��
���ӳɷ�Ӧ��
(5)a. E(CH3COOH)�ǵ���ʣ���a����
b. A(HC��CH)������ԭ�Ӵ���ͬһֱ���ϣ���b����
c. ����ʱ��D(CH3CHO)��������������ͭ����Һ��Ӧ����ש��ɫ��������c��ȷ��
d. 75%(�������)���Ҵ�ˮ��Һ������ҽ����������d��ȷ��
e. ���̶���С���ƿ�Ͷ���Ҵ��У��ƿ���Һ��ײ�������ð���ݣ���e����
��������������cd��

 ����С״Ԫ��������������ϵ�д�
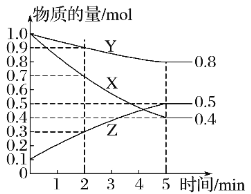
����С״Ԫ��������������ϵ�д�����Ŀ���ס��ҡ����������������У��ס��ҡ�����������ͬ��ij��Ԫ�أ�����֮���ת����ϵ��ͼ��ʾ�������й����ʵ��ƶ���ȷ���ǣ� ��
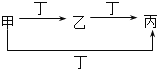
�� | �� | �� | �� | |
A | S | SO2 | SO3 | O2 |
B | CO32- | HCO3- | CO2 | H+ |
C | Cl2 | FeCl3 | FeCl2 | Fe |
D | Al3+ | Al��OH��3 | AlO2- | NH3H2O |
A.AB.BC.CD.D