��Ŀ����
����(Na2CO3)�����������о��й㷺����;��������ʵ����ģ���Ƽ�ԭ����ȡNa2CO3������ͼ��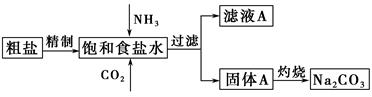
��֪����ʳ��ˮ��ͨ��NH3��CO2�����ķ�ӦΪNaCl��NH3��CO2��H2O=NaHCO3����NH4Cl����ش��������⣺
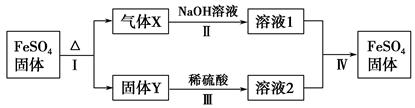
(1)�����к��е�����������Ca2����Mg2����SO42���ȡ�
���Ƴ��ӵIJ���˳����a�� �� �� ��b(����ĸ���)��
a�������ܽ⣬��ȥ����
b�����������pH
c������Ba(OH)2��Һ
d������Na2CO3��Һ
e������
��ʳ��ˮ����ͨ��NH3����ͨ��CO2�������� ��
(2)���չ���A��Na2CO3�� (����ĸ���)�н��С�
a������ b�������� c���ձ� d����ƿ
֤����ҺA�к���NH4���ķ����� ��
����ҺA�����ؽᾧ�ܹ����NH4HCO3����pH��13��Na����K������Һ�м�������NH4HCO3ʹpH���ͣ���Ӧ�����ӷ���ʽΪ ��
(3)��ͼװ���г�����ʵ�����Ʊ�CO2���� (����ĸ���)����bװ���Ʊ�NH3����Һ©����ʢ�ŵ��Լ��� (���Լ�����)����ƿ�ڿɼ���Ĺ����Լ��� (���Լ�����)��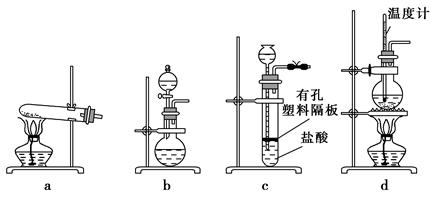
(4)һ����Ȼ���ɷ���aNa2CO3��bNa2SO4��cH2O��ijͬѧ���������ṩ���Լ�����������¼����ⶨNa2CO3������������ʵ�鷽����(������ѡ)���ʵ�鷽����ȫ��
��ѡ����Լ���1.0 mol��L��1 H2SO4��Һ��1.0 mol��L��1 BaCl2��Һ��ϡ��ˮ����ʯ�ҡ�Ca(OH)2��Һ������ˮ
�ٳ�ȡm1g��Ȼ�����Ʒ��������������ˮ�С�
�� ��
�� ��
�ܼ�����Ȼ����к�Na2CO3������������
(1)c��d��e��NH3������ˮ�������������ܽ�����CO2
(2)a��ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤��A�к���NH4����NH4����HCO3����2OH��=NH3��H2O��CO32����H2O
(3)bc��Ũ��ˮ����ʯ��(��NaOH������ʯ��)
(4)�ڼ�������ϡ���Ტ�ȡ�����������ͨ������Ca(OH)2��Һ���۹��ˡ�ϴ�ӡ������������
����

��ͬ������������Һ����ȫ����ʱ����Һ��pH��ͬ��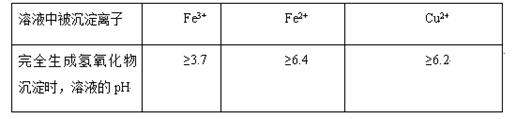
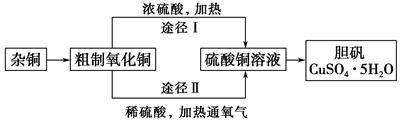
�Ȼ�ͭ���壨CuCl2��2H2O���к�FeCl2���ʣ�Ϊ�Ƶô����Ȼ�ͭ���壬���Ƚ����Ƴ�ˮ��Һ��Ȼ��������ʾ�IJ�����������ᴿ��
��1���������������ʺ���������X���� ������ţ���
| A��NaClO | B��H2O2 | C��KMnO4 | D��Cl2 |
��3��д���ӣ�2������ѡһ������Y���뷴Ӧ���ɳ���Z���ӷ���ʽ�� ��
��4������ܲ���ֱ�������ᾧ�õ�CuCl2��2H2O����? (��ܡ����ܡ�)������,���ûش�;������,�ش����β���? ��
��5��������ͭ��������Ksp��c��Cu2������ c2��OH������2��10��20mol2��L��2��ij����ͭ��Һ��c��Cu2������0��02mol/L����Ҫ����Cu��OH��2������Ӧ������ҺpHʹ֮���� ��