��Ŀ����
�Ĵ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�)��
����д���пհף�
(1)��֪0.5 mol������0.5 molˮ������t �桢p kPaʱ����ȫ��Ӧ����һ��
��̼������(�ϳ���)��������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�______________________��
(2)�ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪���������������û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�
________________________________________________________________��
(3)������ϳɰ�����ת����Ϊ75%ʱ����5.60��107 L����Ϊԭ���ܹ��ϳ�________L������(����������ڱ�״���²ⶨ)
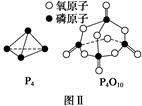
(4)��֪���صĽṹ��ʽΪ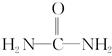 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
��__________________�� ��_________________��
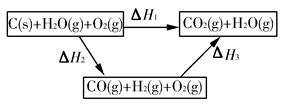
(1)CH4(g)��H2O(g)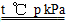 CO(g)��3H2(g) ��H����2a kJ��mol��1
CO(g)��3H2(g) ��H����2a kJ��mol��1
(2)��������������Ũ������������Ӧ���ʣ���С������Ũ�ȡ���������������Ũ�Ⱦ�������ƽ��������Ӧ�����ƶ�
(3)8.4��107
(4)��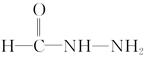 ����NH4N��C��O
����NH4N��C��O
����

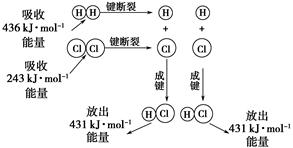
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д���ο�����ͼ������֪E1��134 kJ��mol��1��E2��368 kJ��mol��1������Ҫ��ش����⣺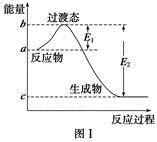
(1)ͼ����1 mol NO2(g)��1 mol CO(g)��Ӧ����CO2��NO�����е������仯ʾ��ͼ�����ڷ�Ӧ��ϵ�м����������Ӧ��������E1�ı仯�� (���������С�����䡱����ͬ)����H�ı仯�� ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ�� ��
(2)�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧ���Ȼ�ѧ����ʽ���£�
��CH3OH(g)��H2O(g)===CO2(g)��3H2(g) ��H����49.0 kJ��mol��1
��CH3OH(g)��O2(g)===CO2(g)��2H2(g) ��H����192.9 kJ��mol��1
��֪��H2O(g)===H2O(l) ��H����44 kJ��mol��1����״�����ȼ��ΪҺ̬ˮ���Ȼ�ѧ����ʽΪ ��
(3)�����ʾ�Dz��ֻ�ѧ���ļ��ܲ�����
| ��ѧ�� | P��P | P��O | O===O | P===O |
| ����/kJ��mol��1 | a | b | c | x |
��֪����ȼ����Ϊd kJ��mol��1����������ȫȼ�յIJ���Ľṹ��ͼ����ʾ�������x�� kJ��mol��1(�ú�a��b��c��d�Ĵ�����ʽ��ʾ)��
��֪��ѧ����������ʽ���ܿ����ת������д�±��Ŀհ�:
| ��ѧ��Ӧ����ʽ(����) | ����ת����ʽ |
| �� | �ɻ�ѧ��ת��Ϊ���� |
��Pb+PbO2+2H2SO4 2PbSO4+2H2O 2PbSO4+2H2O | |
��CaCO3 CaO+CO2�� CaO+CO2�� | |
������Ӧ������������ԭ��Ӧ����(�����)����
(1)�״���һ����Ҫ�Ļ�����Ʒ�������ü״��������Ʊ���ȩ����ȩ����̬�״�ת����������ϵ��ͼ��ʾ��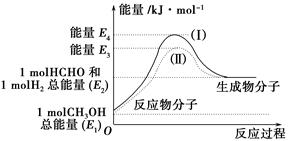
��Ӧ�����е�������ϵ
�ټ״�������ת��Ϊ��ȩ�ķ�Ӧ��________(����ȡ����ȡ�)��Ӧ��
�ڹ��̢�����̢�ķ�Ӧ���Ƿ���ͬ��____________ԭ����____________ ______________________________��
��д���״�������ת��Ϊ��ȩ���Ȼ�ѧ��Ӧ����ʽ______________ _____________________��
(2)��֪����CH3OH(g)��H2O(g)=CO2(g)��3H2(g)����H����49.0 kJ��mol��1
��CH3OH(g)��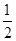 O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
����˵����ȷ����________��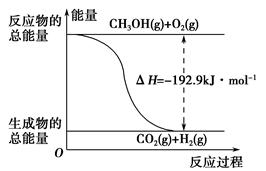
| A��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| B���ٷ�Ӧ�У���Ӧ�������������������������� |
C�����ݢ���֪��Ӧ��CH3OH(l)��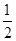 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 |
| D����Ӧ�ڵ������仯��ͼ��ʾ |

 Fe3����3H2O��ƽ�ⳣ��K�� ��
Fe3����3H2O��ƽ�ⳣ��K�� �� CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��