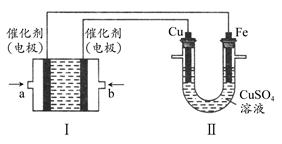
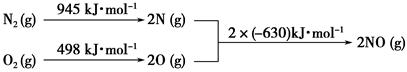
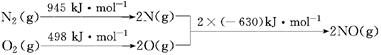
��Ŀ����
��֪���ٽ�úת��Ϊˮú������Ҫ��ѧ��ӦΪC(s)��H2O(g)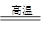 CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
C(s)��O2(g)=CO2 (g)����H����393.5 kJ��mol��1
CO(g)��1/2O2(g)=CO2(g)����H����283.0 kJ��mol��1
H2(g)��1/2O2(g)=H2O(g)����H����242.0 kJ��mol��1
��ش�
(1)����������Ϣ��д��CO��ˮ������Ӧ���Ȼ�ѧ����ʽ��____________________________��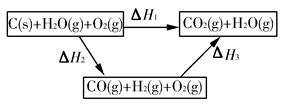
(2)��ͼ�Ǹ��ݸ�˹����������ѭ��ͼ������ͼ��ת����ϵ���Ȼ�ѧ����ʽ���㦤H3��________kJ/mol��
��ȽϦ�H1�릤H3��ֵ�Ƿ����˵����ˮú����ȼ��Ҫ��ֱ��ȼú�ų���������________(�ǻ��)ԭ����___________________________________��
(3)Ŀǰú�����仹��Ҫ����·�����·���䣬��������ѧ֪ʶ���������������·��·����ķ�����____________________________��
(1)CO(g)��H2O(g)=CO2(g)��H2(g)����H����41.0 kJ/mol
(2)���ݸ�˹����û�м��㦤H2���յ�����
(3)��ú��Ϊˮú����ܵ�����
����

��CO2Ϊ̼Դ��ȡ��̼�л����Ϊ�����о����㣬����ΪCO2������ȡ��̼��������ѧ���ݣ�
��Ӧ�� CO2(g)+3H2(g) CH3OH(g)+H2O(g) ?H = ��49��0 kJ��mol-1
CH3OH(g)+H2O(g) ?H = ��49��0 kJ��mol-1
��Ӧ��2CO2(g)+6H2(g) CH3CH2OH(g)+3H2O(g) ?H = ��173��6 kJ��mol-1
CH3CH2OH(g)+3H2O(g) ?H = ��173��6 kJ��mol-1
��1��д����CH3OH(g)�ϳ�CH3CH2OH(g)���Ȼ�ѧ��Ӧ����ʽ��
��2���Է�Ӧ����һ���¶��·�Ӧ�ﵽƽ��ı�־�� ��ѡ����)
a����Ӧ�ﲻ��ת��Ϊ������ b��ƽ�ⳣ��K��������
c��CO2��ת���ʲ������� d����������ƽ����Է����������ٸı䡡
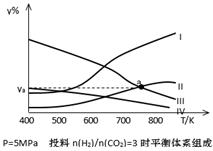
��3�����ܱ������У���Ӧ����һ�������ﵽƽ������������㶨�������CO2ת���ʵĴ�ʩ�� ��ѡ���ţ�
| A�������¶� | B������CO2 | C��������� | D����ȥ�״� |
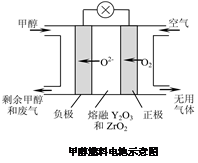
(5)һ���Լ״���ȼ�ϵĵ��ʾ��ͼ��ͼ��д���õ�طŵ�ʱ�����ĵ缫��Ӧʽ�� ��
̼�͵��Ļ���������������������������ء�
��1����һ���¡������ܱ������з�����Ӧ�� Ni(s)+4CO(g)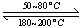 Ni(CO)4(g)��
Ni(CO)4(g)�� H<0�����ø÷�Ӧ���Խ�����ת��Ϊ���ȴ�99��9���ĸߴ������Ը÷�Ӧ��˵����ȷ���� (����ĸ���)��
H<0�����ø÷�Ӧ���Խ�����ת��Ϊ���ȴ�99��9���ĸߴ������Ը÷�Ӧ��˵����ȷ���� (����ĸ���)��
| A������Ni���������CO��ת���ʣ�Ni��ת���ʽ��� |
B����С�����ݻ���ƽ�����ƣ� H��С H��С |
| C����Ӧ�ﵽƽ�����CO�ٴδﵽƽ��ʱ��CO������������� |
| D����4v[Ni(CO)4]=v(CO)ʱ�������л�������ܶȲ���ʱ������˵����Ӧ�Ѵﻯѧƽ��״̬ |
��֪��C(s)+
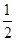 O2(g)=CO(g)
O2(g)=CO(g)  H=-Q1 kJ
H=-Q1 kJ mol-1
mol-1C(s)+ O2(g)=CO2(g)
 H=-Q2 kJ
H=-Q2 kJ mol-1
mol-1S(s)+O2(g)=SO2(g)
 H=-Q3 kJ
H=-Q3 kJ mol-1
mol-1��SO2(g)+2CO(g)=S(s)+2CO2(g)
 H= ��
H= ����3������������ɱ�һ����̼��ԭ���ɽ������ʺͶ�����̼��ͼ28��3�������ֽ��������Cr2O3��SnO2��PbO2��Cu2O)��һ����̼��ԭʱ
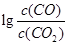 ���¶ȣ�t���Ĺ�ϵ����ͼ��
���¶ȣ�t���Ĺ�ϵ����ͼ��700oCʱ���������ѱ���ԭ�Ľ����������� (�ѧʽ)����һ����̼��ԭ�ý���������ʱ������Ӧ����ʽϵ��Ϊ��������ȣ��÷�Ӧ��ƽ�ⳣ��(K)��ֵ���� ��
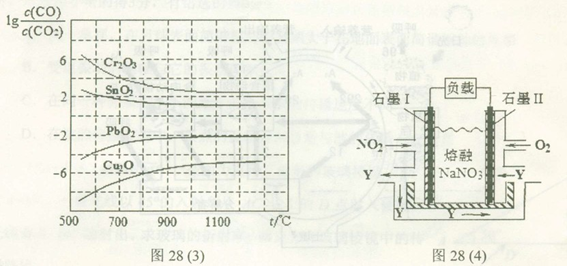
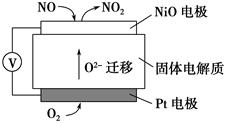
��4��NO2��O2������NaNO3������ȼ�ϵ�أ���ԭ������ͼ28��4����ʾ���õ����ʹ�ù�����ʯīI�缫������������Y����缫��ӦʽΪ ��
����ȼ�ϵ��ʹ��һ��ʱ����ռ���20mol Y������������Ҫ���ı�״�������������Ϊ L��
�ϳ����������о�����Ҫ���⣬Ŀǰ��ҵ�ϳɰ���ԭ��Ϊ��
N2(g)+3H2(g)  2NH3(g) ��H=-93��0kJ/mol
2NH3(g) ��H=-93��0kJ/mol
��1��ij�¶��£���2 L�ܱ������з���������Ӧ�������������
| ʱ��/h ���ʵ���/mol | 0 | 1 | 2 | 3 | 4 |
| N2 | 2��0 | 1��83 | 1��7 | 1��6 | 1��6 |
| H2 | 6��0 | 5��49 | 5��1 | 4��8 | 4��8 |
| NH3 | 0 | 0��34 | 0��6 | 0��8 | 0��8 |
��0~2 h�ڣ�v��N2��= ��
��ƽ��ʱ��H2��ת����Ϊ____�����¶��£���Ӧ2NH3(g)
 N2(g)+3H2(g)��ƽ�ⳣ��K= ��
N2(g)+3H2(g)��ƽ�ⳣ��K= �����������¶Ⱥ�������䣬��ʼͶ���N2��H2��NH3�����ʵ����ֱ�Ϊa mol��b mol��c mol���ﵽƽ���NH3�ȵ�Ũ�����ϱ�����ͬ��Ϊ ����ѡ����ĸ����
A��a=l��b=3��c=0 B��a=4��b=12��c=0
C��a=0��b=0��c=4 D��a=l��b=3��c=2
��2�����ݱ��������¡���ѹ�£�N2�ڲ��������������Ķ������Ѵ�����������ˮ������Ӧ������NH3��O2����֪��H2��ȼ���Ȧ�H=-286kJ/mol��������NH3��Ӧ���Ȼ�ѧ����ʽΪ ��
��3�����ø����ӵ����Ե�SCY�մɣ��ܴ���H'����ͨ����ⷨҲ�ɺϳɰ���ԭ��Ϊ��
N2(g)+3H2(g)
 2NH3(g)���ڵ�ⷨ�ϳɰ��Ĺ����꣬Ӧ��N2���ϵ�ͨ�� ___�����õ缫��ӦʽΪ ��
2NH3(g)���ڵ�ⷨ�ϳɰ��Ĺ����꣬Ӧ��N2���ϵ�ͨ�� ___�����õ缫��ӦʽΪ �� 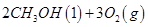 =
= mol
mol
 =
=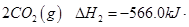 mol
mol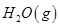 =
=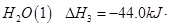 mol
mol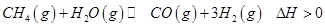
 ��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa����
��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa����
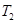 (�<������>����="����ͬ)��A��B��C���㴦��Ӧƽ�ⳣ��(
(�<������>����="����ͬ)��A��B��C���㴦��Ӧƽ�ⳣ��(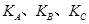 )�Ĵ�С��ϵΪ ��
)�Ĵ�С��ϵΪ ��
 ͨ���ݻ�Ϊ1L�Ķ����ܱ������з�����Ӧ����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� (�����)��
ͨ���ݻ�Ϊ1L�Ķ����ܱ������з�����Ӧ����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� (�����)��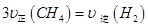
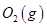 ��KOH(aq)��Ƴ���ͼb��ʾ�ĵ��װ�ã���õ�ظ����ĵ缫��ӦʽΪ ��
��KOH(aq)��Ƴ���ͼb��ʾ�ĵ��װ�ã���õ�ظ����ĵ缫��ӦʽΪ ��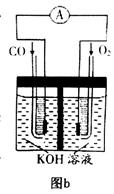

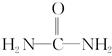 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��