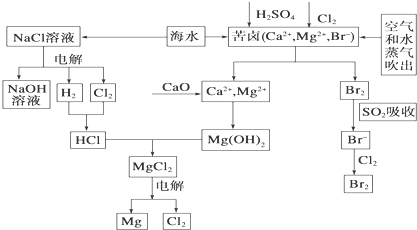
��Ŀ����
2��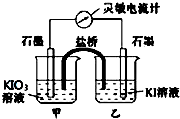 ����ӦIO3-+5I-+6H+?3I2+3H2O��Ƴ���ͼ��ʾ��ԭ��أ�
����ӦIO3-+5I-+6H+?3I2+3H2O��Ƴ���ͼ��ʾ��ԭ��أ���1����ʼʱ����ձ��м�������Ũ���ᣬ������ָ������ƫת����ʱ�׳��з����ĵ缫��ӦʽΪ2IO3-+10e-+12H+=I2+6H2O�����������й��ڵ����ƵĶ���������˵����ȷ����a��b�����ţ�
a�������ƶ�����С b�������ƶ����п��ܱ�Ϊ0
c�������ƶ���һֱ���� d�������ƵĶ���������
��2������ڼ�Ũ����ǰ���ס����ձ��ж����������Һ������Һ�������ձ��Ǽס��ң���ס������ҡ�����
��3������һ��ʱ������������ձ�����ŨNaOH��Һ����ʱ�ҳ��з����ĵ缫��ӦʽΪI2+2e-=2I-��������ָ������������ҡ���ƫת��
���� ��ʼ��������Ũ����ʱ������I-ʧ��������I2������Ϊ����������IO3-�õ�������I2�����Ϊ���������Լ��������Һ�������ձ��о�����ɫ�����ӴӸ������������������Ҿ���������ף�
������ձ��е��뼸��ŨNaOH��Һ�����I2��ʧ���ӱ���������IO3-����Ϊ��������Ϊ�������Դ˽����⣮
��� �⣺��1������IO3-�õ�������I2��Ϊԭ��ص��������缫����ʽΪ2IO3-+10e-+12H+=I2+6H2O��
���ŷ�Ӧ�Ľ��У���ҺŨ����С��������ĵ�����С�������ƶ�����С���÷�ӦΪ���淴Ӧ������Ӧ�ﵽƽ��״̬ʱ�������ʵ�Ũ�Ȳ��ٸı䣬��û�е���ͨ�������ƣ����Ե����ƶ���Ϊ�㣻��a��b��ȷ��
�ʴ�Ϊ��2IO3-+10e-+12H+=I2+6H2O��a��b��
��2������I-ʧ���ӷ���������Ӧ����I2������IO3-�õ��ӷ�����ԭ��Ӧ����I2������������Һ�������ձ��о�����ɫ���ʴ�Ϊ���ס��ң�
��3��������ձ��е��뼸��ŨNaOH��Һ�����I2��ʧ���ӱ���������IO3-����Ϊ��������Ϊ����������������ԭ��Ӧ���缫����ʽΪI2+2e-=2I-������������෴���������ƫ����
�ʴ�Ϊ��I2+2e-=2I-����
���� ���⿼��ԭ��أ�Ϊ�߿��������ͣ�������ѧ���ķ��������Ŀ��飬ע������������ԭ��Ӧ�����������ĵ缫��Ӧ����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 7.80g Na2O2��5.85g NaCl��������������Ϊ0.1NA | |
| B�� | һ�������£�1.4g N2��0.2mol H2��ϳ�ַ�Ӧ��ת�Ƶĵ�����Ϊ0.3NA | |
| C�� | ���³�ѹ�£�22.4LNH3����������С��NA | |
| D�� | ��״���£�28g����ϩ��ȫȼ�գ�������CO2��Ϊ2NA |
 �Ҷ����׳Ʋ�����һ�ֶ�Ԫ���ᣨ�ṹ��ʽΪHOOC-COOH���ɼ�дΪH2C2O4��������һ����Ҫ�Ļ���ԭ�ϣ���������0.01mol/L��H2C2O4��KHC2O4��K2C2O4��Һ��pH���±���ʾ����գ�
�Ҷ����׳Ʋ�����һ�ֶ�Ԫ���ᣨ�ṹ��ʽΪHOOC-COOH���ɼ�дΪH2C2O4��������һ����Ҫ�Ļ���ԭ�ϣ���������0.01mol/L��H2C2O4��KHC2O4��K2C2O4��Һ��pH���±���ʾ����գ�| H2C2O4 | KHC2O4 | K2C2O4 | |
| pH | 2.1 | 3.1 | 8.1 |
��2��KHC2O4��Һ�����Ե�ԭ����HC2O4-�ĵ���̶ȴ���ˮ��̶���0.1mol/L�IJ��������Һ��μ�NaOH��Һ�����ԣ���ʱ��Һ�������Ũ�ȹ�ϵ��ȷ����AD
A��c��K+���Tc��HC2O4-��+c��H2C2O4��+c��C2O42-�� B��c��Na+���Tc��H2C2O4��+c��C2O42-��
C��c��K+��+c��Na+���Tc��HC2O4-��+c��C2O42-�� D��c��K+����c��Na+��
��3��H2C2O4�����Ը��������Һ��Ӧ�������������ݣ�CO2����������ɫ��ʧ��д����Ӧ�����ӷ���ʽ2MnO4-+5H2C2O4+6H+��2Mn2++10CO2��+8H2O��֪�÷�Ӧ��ʼʱ���ʽ����������ӿ죬���ܵ�ԭ�������ɵ�Mn2+�Ը÷�Ӧ���д�����
��4��ijͬѧ���ʵ����ͼ��ʾ�������ձ��е��Թܶ��ֱ�ʢ��2ml0.1mol/LH2C2O4��Һ��4mL 0.1mol/L ����KMnO4��Һ���ֱ��ϲ�����¼��Һ��ɫ����ʱ�䣮��ʵ��Ŀ�����о��¶ȶԷ�Ӧ���ʵ�Ӱ�죬����ʵ��ʼ��û�п�����Һ��ɫ���Ʋ�ԭ��KMnO4��Һ������
| A�� | ���ȵĴ�����ϴ���� | B�� | ����������NaCl��Һ�� | ||
| C�� | �����ľ�ˮ���� | D�� | ��ĭ�������ʹ��ԭ�� |
| A�� | bһ��С��c | B�� | ���ʵ�������Z��Y | ||
| C�� | Y2-�Ļ�ԭ�Դ���Z- | D�� | X��Y�ɴ���ͬ���ڻ�X��Y�������� |
| A�� | CH3CH��Br��OH | B�� | CH3CH��CH2OH��CHO | ||
| C�� | CH3CH2CH��CH3��CH2CH3 | D�� | HOOCCH��Br��COOCH3 |
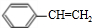 ��A�����д���ͬһƽ���ԭ�������16����
��A�����д���ͬһƽ���ԭ�������16����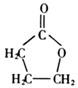 ��
��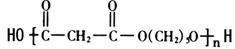 ��
��