��Ŀ����
�������գ���1�������а������ʣ���MgCl2����ڸɱ���NaOH����ܰ��ף�P4������ݽ��ʯ�����壮�����������Ӿ������______�����ڷ��Ӿ������______������ԭ�Ӿ������______��
��2�������з��ӣ���CO2��NO2��SO3��BF3��NH3���������ڷǼ��Է��ӵ���______�����ӵ�VSEPRģ��Ϊƽ�������ε���______�����ڷ��ӵ����幹��Ϊ�����ε���______��
��3������ͬ�����£�SO2��ˮ�е��ܽ�Ⱥ�CO2��ˮ�е��ܽ����ȣ�SO2���ܽ�ȴ���ӷ������ʵĽǶȲ�������______
��4���ԱȽ�ͬ����Ԫ�ص��⻯��H2O��H2S��H2Se���ȶ��Ժͷе�ߵͣ���˵�����ɣ�
�ȶ��ԣ�______ ���ɣ�______��
�е㣺______ ���ɣ�______��
���𰸡���������1���������ʵķ����жϾ�������һ��Ϊ�����������������K2O��Na2O2�ȣ���ǿ���NaOH��KOH�ȣ��;�����������������Ӿ��壮������ǽ������ʣ������ʯ��ʯī������衢�������⣩����̬�⻯��ǽ����������SiO2�⣩���ᡢ��������л�����л����⣩���Ƿ��Ӿ��壮������ԭ�Ӿ��嵥���н��ʯ������衢������ȣ�������ԭ�Ӿ��廯������̼���衢��������ȣ��������ʣ������⣩��Ͻ��ǽ������壮
��2�����ݷ��ӵĿռ�ṹ�жϷ��Ӽ��ԣ�������������غ�Ϊ�Ǽ��Է��ӣ����غ�Ϊ���Է��ӣ�VSEPRģ��Ϊƽ������������ԭ�ӵļ۲���Ӷ�����3�����幹��Ϊ�����Σ�����ԭ�ӵļ۲���Ӷ�����4������1�Թ¶Ե��Ӷԣ�
��3��ˮ�Ǽ��Է��ӣ�������������ԭ���жϣ�
��4���ǽ�����Խǿ���⻯��Խ�ȶ���H2O����֮�����������е���ߣ�H2Se��H2S��ṹ���ƣ���Է�������Խ���Ӽ��������е�Խ�ߣ�
����⣺��1���������Ӿ�����ǣ�MgCl2���塢��NaOH���壻���ڷ��Ӿ�����ǣ��ڸɱ��ۡ��ܰ��ף�P4�����壻����ԭ�Ӿ�����ǣ��ݽ��ʯ�����壮
�ʴ�Ϊ���٢ۣ��ڢܣ��ݢޣ�
��2����CO2��Cԭ�ӵļ۲���Ӷ�Ϊ2��VSEPRģ��Ϊֱ���ͣ�û�й¶Ե��Ӷԣ�����Ϊֱ���ͶԳƽṹ�����ڷǼ��Է��ӣ�
��NO2��Nԭ�ӵļ۲���Ӷ�Ϊ =2.5����3������VSEPRģ��Ϊƽ�������Σ�Nԭ����δ�ɼ��ĵ��ӣ�����ΪV�Σ����ڼ��Է��ӣ�
=2.5����3������VSEPRģ��Ϊƽ�������Σ�Nԭ����δ�ɼ��ĵ��ӣ�����ΪV�Σ����ڼ��Է��ӣ�
��SO3��Sԭ�ӵļ۲���Ӷ�Ϊ =3��VSEPRģ��Ϊƽ�������Σ�Sԭ��û�й¶Ե��Ӷԣ�����Ϊƽ�������Σ��ṹ�Գƣ����ڷǼ��Է��ӣ�
=3��VSEPRģ��Ϊƽ�������Σ�Sԭ��û�й¶Ե��Ӷԣ�����Ϊƽ�������Σ��ṹ�Գƣ����ڷǼ��Է��ӣ�
��BF3��Bԭ�ӵļ۲���Ӷ�Ϊ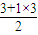 =3��VSEPRģ��Ϊƽ�������Σ�Bԭ��û�й¶Ե��Ӷԣ�����Ϊƽ�������Σ��ṹ�Գƣ����ڷǼ��Է��ӣ�
=3��VSEPRģ��Ϊƽ�������Σ�Bԭ��û�й¶Ե��Ӷԣ�����Ϊƽ�������Σ��ṹ�Գƣ����ڷǼ��Է��ӣ�
��NH3��Nԭ�ӵļ۲���Ӷ�Ϊ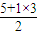 =4��VSEPRģ��Ϊ�������壬Nԭ����1�Թ¶Ե��Ӷԣ�����Ϊ�����ͣ��ṹ���Գƣ����ڷǼ��Է��ӣ�
=4��VSEPRģ��Ϊ�������壬Nԭ����1�Թ¶Ե��Ӷԣ�����Ϊ�����ͣ��ṹ���Գƣ����ڷǼ��Է��ӣ�
�����������ڷǼ��Է��ӵ��Ǣ٢ۢܣ����ӵ�VSEPRģ��Ϊƽ�������ε��Ǣڢۢܣ����ӵ����幹��Ϊ�����ε��Ǣݣ�
�ʴ�Ϊ���٢ۢܣ��ڢۢܣ��ݣ�
��3��SO2��Sԭ�ӵļ۲���Ӷ�Ϊ =3��VSEPRģ��Ϊƽ�������Σ�Sԭ��1�Թ¶Ե��Ӷԣ�����ΪV�Σ��ṹ���Գƣ����ڼ��Է��ӣ�CO2�ǷǼ��Է��ӣ�ˮ�Ǽ��Է��ӣ�������������ԭ����SO2��ˮ�е��ܽ�ȱ�CO2�Ĵ�
=3��VSEPRģ��Ϊƽ�������Σ�Sԭ��1�Թ¶Ե��Ӷԣ�����ΪV�Σ��ṹ���Գƣ����ڼ��Է��ӣ�CO2�ǷǼ��Է��ӣ�ˮ�Ǽ��Է��ӣ�������������ԭ����SO2��ˮ�е��ܽ�ȱ�CO2�Ĵ�
�ʴ�Ϊ��SO2�Ǽ��Է��ӣ�CO2�ǷǼ��Է��ӣ�ˮ�Ǽ��Է��ӣ�������������ԭ����SO2��ˮ�е��ܽ�ȱ�CO2�Ĵ�
��4���ǽ�����Խǿ����̬������Խ�ȶ����ǽ�����O��S��Se�������ȶ��ԣ�H2O��H2S��H2Se��
H2O���γɷ��Ӽ�������е���ߣ�H2Se��Է���������H2S���Ӽ������������H2Se��H2S�е�ߣ����Էе㣺H2O��H2Se��H2S��
�ʴ�Ϊ��H2O��H2S��H2Se���ǽ�����Խǿ����̬������Խ�ȶ����ǽ�����O��S��Se��
H2O��H2Se��H2S��H2O���γɷ��Ӽ�������е���ߣ�H2Se��Է���������H2S���Ӽ������������H2Se��H2S�е�ߣ�
���������龧�����͡��۲���ӶԻ������ۡ����Ӽ�������������ԭ���������ͬ����Ԫ�����ʵݱ���ɵȣ��ۺ��Խϴ��Ѷ��еȣ��Ƕ�֪ʶ��ֱ�����ã���Ҫѧ��������ʵ�Ļ�����
��2�����ݷ��ӵĿռ�ṹ�жϷ��Ӽ��ԣ�������������غ�Ϊ�Ǽ��Է��ӣ����غ�Ϊ���Է��ӣ�VSEPRģ��Ϊƽ������������ԭ�ӵļ۲���Ӷ�����3�����幹��Ϊ�����Σ�����ԭ�ӵļ۲���Ӷ�����4������1�Թ¶Ե��Ӷԣ�
��3��ˮ�Ǽ��Է��ӣ�������������ԭ���жϣ�
��4���ǽ�����Խǿ���⻯��Խ�ȶ���H2O����֮�����������е���ߣ�H2Se��H2S��ṹ���ƣ���Է�������Խ���Ӽ��������е�Խ�ߣ�
����⣺��1���������Ӿ�����ǣ�MgCl2���塢��NaOH���壻���ڷ��Ӿ�����ǣ��ڸɱ��ۡ��ܰ��ף�P4�����壻����ԭ�Ӿ�����ǣ��ݽ��ʯ�����壮
�ʴ�Ϊ���٢ۣ��ڢܣ��ݢޣ�
��2����CO2��Cԭ�ӵļ۲���Ӷ�Ϊ2��VSEPRģ��Ϊֱ���ͣ�û�й¶Ե��Ӷԣ�����Ϊֱ���ͶԳƽṹ�����ڷǼ��Է��ӣ�
��NO2��Nԭ�ӵļ۲���Ӷ�Ϊ
 =2.5����3������VSEPRģ��Ϊƽ�������Σ�Nԭ����δ�ɼ��ĵ��ӣ�����ΪV�Σ����ڼ��Է��ӣ�
=2.5����3������VSEPRģ��Ϊƽ�������Σ�Nԭ����δ�ɼ��ĵ��ӣ�����ΪV�Σ����ڼ��Է��ӣ���SO3��Sԭ�ӵļ۲���Ӷ�Ϊ
 =3��VSEPRģ��Ϊƽ�������Σ�Sԭ��û�й¶Ե��Ӷԣ�����Ϊƽ�������Σ��ṹ�Գƣ����ڷǼ��Է��ӣ�
=3��VSEPRģ��Ϊƽ�������Σ�Sԭ��û�й¶Ե��Ӷԣ�����Ϊƽ�������Σ��ṹ�Գƣ����ڷǼ��Է��ӣ���BF3��Bԭ�ӵļ۲���Ӷ�Ϊ
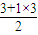 =3��VSEPRģ��Ϊƽ�������Σ�Bԭ��û�й¶Ե��Ӷԣ�����Ϊƽ�������Σ��ṹ�Գƣ����ڷǼ��Է��ӣ�
=3��VSEPRģ��Ϊƽ�������Σ�Bԭ��û�й¶Ե��Ӷԣ�����Ϊƽ�������Σ��ṹ�Գƣ����ڷǼ��Է��ӣ���NH3��Nԭ�ӵļ۲���Ӷ�Ϊ
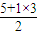 =4��VSEPRģ��Ϊ�������壬Nԭ����1�Թ¶Ե��Ӷԣ�����Ϊ�����ͣ��ṹ���Գƣ����ڷǼ��Է��ӣ�
=4��VSEPRģ��Ϊ�������壬Nԭ����1�Թ¶Ե��Ӷԣ�����Ϊ�����ͣ��ṹ���Գƣ����ڷǼ��Է��ӣ������������ڷǼ��Է��ӵ��Ǣ٢ۢܣ����ӵ�VSEPRģ��Ϊƽ�������ε��Ǣڢۢܣ����ӵ����幹��Ϊ�����ε��Ǣݣ�
�ʴ�Ϊ���٢ۢܣ��ڢۢܣ��ݣ�
��3��SO2��Sԭ�ӵļ۲���Ӷ�Ϊ
 =3��VSEPRģ��Ϊƽ�������Σ�Sԭ��1�Թ¶Ե��Ӷԣ�����ΪV�Σ��ṹ���Գƣ����ڼ��Է��ӣ�CO2�ǷǼ��Է��ӣ�ˮ�Ǽ��Է��ӣ�������������ԭ����SO2��ˮ�е��ܽ�ȱ�CO2�Ĵ�
=3��VSEPRģ��Ϊƽ�������Σ�Sԭ��1�Թ¶Ե��Ӷԣ�����ΪV�Σ��ṹ���Գƣ����ڼ��Է��ӣ�CO2�ǷǼ��Է��ӣ�ˮ�Ǽ��Է��ӣ�������������ԭ����SO2��ˮ�е��ܽ�ȱ�CO2�Ĵ��ʴ�Ϊ��SO2�Ǽ��Է��ӣ�CO2�ǷǼ��Է��ӣ�ˮ�Ǽ��Է��ӣ�������������ԭ����SO2��ˮ�е��ܽ�ȱ�CO2�Ĵ�
��4���ǽ�����Խǿ����̬������Խ�ȶ����ǽ�����O��S��Se�������ȶ��ԣ�H2O��H2S��H2Se��
H2O���γɷ��Ӽ�������е���ߣ�H2Se��Է���������H2S���Ӽ������������H2Se��H2S�е�ߣ����Էе㣺H2O��H2Se��H2S��
�ʴ�Ϊ��H2O��H2S��H2Se���ǽ�����Խǿ����̬������Խ�ȶ����ǽ�����O��S��Se��
H2O��H2Se��H2S��H2O���γɷ��Ӽ�������е���ߣ�H2Se��Է���������H2S���Ӽ������������H2Se��H2S�е�ߣ�
���������龧�����͡��۲���ӶԻ������ۡ����Ӽ�������������ԭ���������ͬ����Ԫ�����ʵݱ���ɵȣ��ۺ��Խϴ��Ѷ��еȣ��Ƕ�֪ʶ��ֱ�����ã���Ҫѧ��������ʵ�Ļ�����

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ