��Ŀ����
7����ҵ���Ի�ͭ����Ҫ�ɷ�CuFeS2��Ϊԭ���Ʊ�CuSO4•5H2O����Ҫ�������£�
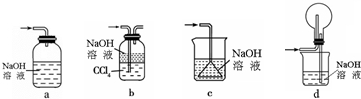
��1������װ�ÿ�������������X����bd������ţ���
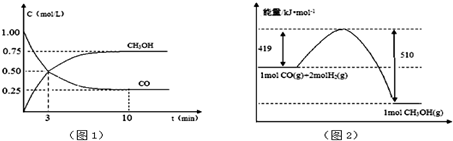
��2��ij�о���ѧϰС������ͭ��CO��Ӧ����ȡ��ͭ��
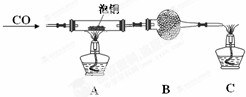
��װ��B�е�ҩƷΪ��ʯ�ң�
��ʵ��ʱ�����ν������²�������װ���������װ�õ������ԡ���װҩƷ��ͨ�����塢�ռ�CO���鴿�ȡ���ȼ�ƾ��ƣ�
��3������Y�ijɷ�ΪFe2O3��FeO��ѡ���ṩ���Լ������ʵ����֤�����к���FeO��д���й�ʵ���������������ۣ��ṩ���Լ���ϡ���ᡢϡ���ᡢKSCN��Һ��KMnO4��Һ��NaOH��Һ����ˮ��
ȡ����������������ϡ�����ܽ⣬����Һ�м���KMnO4��Һ������Һ�Ϻ�ɫ��ȥ����֤�������к���FeO��
��4�����ͭ�м������������Ļ�����Һ��ȡ����ͭʱ�����ʲ��μӷ�Ӧ����������H2SO4��HNO3��������ʵ���֮��Ϊ3��2��
��5���õζ����ⶨ���ò�Ʒ��CuSO4•5H2O�ĺ�������ȡa g��Ʒ���100mL��Һ��ȡ��20.00mL����c mol•L-1�ζ��� EDTA��H2Y2-������Һ�ζ����յ㣨�ζ����������ʷ�Ӧ�������ĵζ���b mL���ζ���Ӧ���£�Cu2++H2Y2-=CuY2-+2H+����CuSO4•5H2O��������Ϊ$\frac{125bc}{a}%$���ζ���������ˮϴ�Ӻ�ֱ��ע�����Һ����ᵼ�²ⶨ���ƫ�ߣ�
���� ��1������X�Ƕ�������ѡ���Լ����ն��������ܲ����µ���Ⱦ���壬����������������������ѡ���и����ʵ������Լ��ܽ�ȴ�С�жϣ�
��2���ٴ�ͭ��CO��Ӧ���ɶ�����̼��ʣ��CO�ж�������ȼ�մ������ڴ�֮ǰ����װ��B���ն�����̼���ݴ˽�ɣ�
�����ݸ�ʵ����Ⱥ�˳��شɣ�
��3����Fe2O3�к���FeO������ϡ�ᣨ�������ԣ��ܽ�����ɵ��������ӣ�����л�ԭ�ԣ����������Լ���KMnO4��Һ����ǿ�����ԣ�������KMnO4��Һ��ɫ��֤����
��4�������������ǡ�÷�Ӧʱ�������������ʵ���֮����ѣ��������ӷ���ʽ���㼴�ɣ�
��5�����ݷ�Ӧ����ʽ���ζ����ݼ��������ͭ�����ʵ������ټ��������ͭ�������������������CuSO4•5H2O���������ı���ʽ�������ζ�����Ҫ��ϴ���ݴ˽�ɣ�
��� �⣺��1����������ͼ��֪X�����Ƕ�������ѡ���Լ����ն��������ܲ����µ���Ⱦ���壬
a������δ����Һ�����£��������������ã���a����
b���������ƿ������ն��������Ҷ��������ܽ�Ƚϴ���ͨ�뵽���Ȼ�̼��Һ�У��������������ã��ܷ�ֹ��������b��ȷ��
c�����������ܽ�Ƚϴ��۵�©������Һ�����£����������������ã���c����
d�����۵�Բ����ƿ��ȫƿ�����ã��ܷ�ֹ��������d��ȷ��
��ѡbd��
��2���ٴ�ͭ��CO��Ӧ���ɶ�����̼��ʣ��CO�ж�������ȼ�մ������ڴ�֮ǰ����װ��B���ն�����̼����B��Ӧʢ�ż�ʯ�ң��ʴ�Ϊ����ʯ�ң�
����װ��������Ӧ����װ�õ������ԣ�����CO��ȼ��������ը������ȼǰ���鴿���ʴ�Ϊ������װ�������ԣ��ռ�CO���鴿�ȣ�
��3����Fe2O3�к���FeO������ϡ�ᣨ�������ԣ��ܽ�����ɵ��������ӣ�����л�ԭ�ԣ����������Լ���KMnO4��Һ����ǿ�����ԣ���ʹKMnO4��Һ��ɫ����ѡ���Լ�Ϊϡ���ᡢKMnO4��Һ������Ϊȡ������������ϡ���ᣬȻ��μ�KMnO4��Һ���۲쵽��ҺʹKMnO4��Һ��ɫ����֤������FeO��
�ʴ�Ϊ��ȡ�����������ӹ���ϡ�����ܽ⣬����Һ�м��뼸�θ��������Һ������Һ��ɫ��ȥ����֤�������к���FeO��
��4�������������ǡ�÷�Ӧʱ�������������ʵ���֮����ѣ������ӷ���ʽ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O ��֪����Ӧ������2molNO3-����Ҫ8molH+���������2mol������6mol�������������ṩ��������Ϊ3mol�������������������ʵ���֮��Ϊ3��2���ʴ�Ϊ��3��2��
��5�����ݷ���ʽ��֪20.00mL��Һ��n��CuSO4•5H2O��=n��EDTA��=c��b��10-3mol��
����m��CuSO4•5H2O��=c��b��10-3mol��250g/mol=0.25bc g��
���100mL��Һ��CuSO4•5H2O������Ϊ��0.25bc��5=1.25bcg�����Ԧ�=$\frac{1.25bc}{a}$��100%=$\frac{125bc}{a}%$��
�ζ�����Ҫ����ʢװ��Һ��ϴ�������ϡ����װ��Һ��Ũ�ȣ����½��ƫ�ߣ��ʴ�Ϊ��$\frac{125bc}{a}%$���ߣ�
���� ���⿼����������ķ�����ᴿ���ؼ�����ȡ���е���Ϣ��������ѧ֪ʶ��ɣ������Ѷ��еȣ�

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�| A�� | 70% | B�� | 30% | C�� | 52.4% | D�� | 22.2% |
| A�� | �������Ե��� | B�� | �����ǻ���� | ||
| C�� | �������Ӵ�С��1��100nm֮�� | D�� | ����ķ�ɢ��Ϊ���� |
| A�� | 50 mL 0.1 mol/L��NaCl��Һ | B�� | 100 mL 0.2 mol/L��NaCl��Һ | ||
| C�� | 25 mL 0.2 mol/L��Na2SO4��Һ | D�� | 10 mL 0.5 mol/L��Na2CO3��Һ |
| A�� | CH3CH3��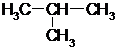 | B�� | CH3CH=CH2�루�����飩 | ||
| C�� | CH3OH��HOCH2CH2OH | D�� | 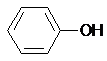 �� ��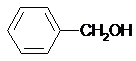 |
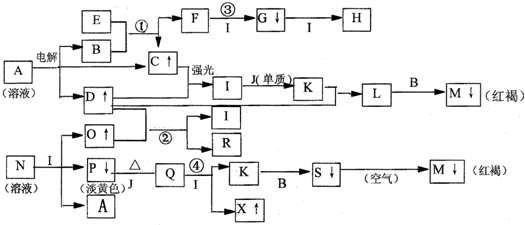
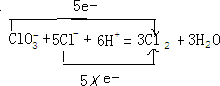 ��
��