��Ŀ����
��10�֣�������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������T��������������������������ȣ���ش��������⣺
��1��T��ԭ�ӽṹʾ��ͼΪ_______________________��
��2��Ԫ�صķǽ�����Ϊ��ԭ�ӵĵõ�����������Q__________R(�ǿ�ڡ������ڡ�)����3�� W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��4��ԭ��������R��1��Ԫ�ص�һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ����ʽ��__________________________________________��
��5��R�ж�����������м���Է���������С����һ�������£�2 L�ļ�������0.5 L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û����������������ɵ�R�ĺ������εĻ�ѧʽ��_______________��
| �� | Q | R | �� |
| T | �� | �� | W |
��2��Ԫ�صķǽ�����Ϊ��ԭ�ӵĵõ�����������Q__________R(�ǿ�ڡ������ڡ�)����3�� W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��4��ԭ��������R��1��Ԫ�ص�һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ����ʽ��__________________________________________��
��5��R�ж�����������м���Է���������С����һ�������£�2 L�ļ�������0.5 L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û����������������ɵ�R�ĺ������εĻ�ѧʽ��_______________��
��10�֣���1�� ��2�����ڡ�
��2�����ڡ�
��3��S + 2H2SO4(Ũ) 3SO2��+ 2H2O
3SO2��+ 2H2O
��4��2H2O2 2H2O + O2�� ��5��NaNO2 ( ÿ��2��)
2H2O + O2�� ��5��NaNO2 ( ÿ��2��)
 ��2�����ڡ�
��2�����ڡ���3��S + 2H2SO4(Ũ)
 3SO2��+ 2H2O
3SO2��+ 2H2O��4��2H2O2
 2H2O + O2�� ��5��NaNO2 ( ÿ��2��)
2H2O + O2�� ��5��NaNO2 ( ÿ��2��) T��������������������������ȣ���T����������Q��C��R��N��W��S��
T��������������������������ȣ���T����������Q��C��R��N��W��S����1������ԭ������Ϊ13��ԭ�ӽṹʾ��ͼΪ ��
��2��ͬ�����������ҷǽ���������ǿ������Q�ķǽ���������R�ġ�
��3���ڼ��������£�Ũ��������������������SO2��ˮ������ʽΪS + 2H2SO4(Ũ)
 3SO2��+ 2H2O��
3SO2��+ 2H2O����4��ԭ��������R��1��Ԫ�ص�һ���⻯��ˮ˫��ˮ���ֽ�����������ˮ������ʽΪ2H2O2
 2H2O + O2�� ��
2H2O + O2�� ����5������Է���������С�������NO��NO�����������ʵ���֮����4�U1�����Ը��ݵ��ʵ�ʧ�غ��֪��NO�����������е�Ԫ�صĻ��ϼ��ǣ�3�ۣ�������NaNO2��

��ϰ��ϵ�д�
�����Ŀ
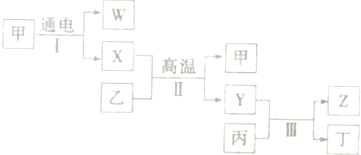
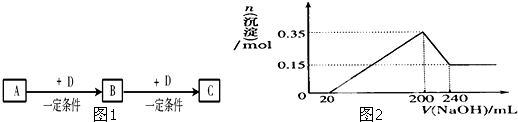
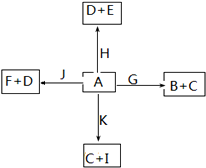
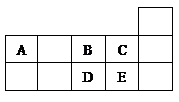
 C��
C�� N����������ͬ����������ͬ
N����������ͬ����������ͬ