��Ŀ����
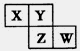
X��Y��Z��W��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ��������Ϣ���±���
��1��Wλ��Ԫ�����ڱ��� ���ڵ� �壬��ԭ��������� �����ӡ�
��2��X�Ļ����Ա�Y�� (�ǿ��������)��X��Y����̬�⻯���У����ȶ����� (д��ѧʽ)��
��3��д��Z2Y2�ĵ���ʽ ��XY2�Ľṹʽ ��
��4����Xԭ������ԭ���γɵĶ��ַ����У���Щ���ӵĺ˴Ź���������ʾ�������⣬д������һ�ַ��ӵ����� ����Ԫ�ء�X��Y��ԭ��Ҳ�ɹ�ͬ�γɶ��ַ��Ӻ�ij�ֳ����������ӣ�д������һ�ַ�������������ӷ�Ӧ�����ӷ���ʽ ��
| Ԫ�� | �����Ϣ |
| X | Xԭ�ӵ�L���������K���������2�� |
| Y | Yԭ�ӵ����������Ų�ʽΪ��nsnnpn+2 |
| Z | Z����������Ϊ23��������Ϊ12��ԭ�Ӻ� |
| W | W�ж��ֻ��ϼۣ����ɫ���������ڿ����л�Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ |
��2��X�Ļ����Ա�Y�� (�ǿ��������)��X��Y����̬�⻯���У����ȶ����� (д��ѧʽ)��
��3��д��Z2Y2�ĵ���ʽ ��XY2�Ľṹʽ ��
��4����Xԭ������ԭ���γɵĶ��ַ����У���Щ���ӵĺ˴Ź���������ʾ�������⣬д������һ�ַ��ӵ����� ����Ԫ�ء�X��Y��ԭ��Ҳ�ɹ�ͬ�γɶ��ַ��Ӻ�ij�ֳ����������ӣ�д������һ�ַ�������������ӷ�Ӧ�����ӷ���ʽ ��
��1���� ��(��1��) 2
��2�� �� H2O
��3��
 O =C=O
O =C=O ��4������(���Ȳ��2-����ϩ��1,2,4,5-�ļ�����) CH3COOH��HCO3���� CH3COO����H2O��CO2����2�֣�
���������
��1��X��C Y��O Z��Na W��Fe �� �� 2
��2��X��C Y��Oͬ���ڷǽ�������ǿ��C <O����X< Y���ǽ�����ǿ����̬�⻯���ȶ���H2O
��3��Z2Y2��Na2O2��XY2��CO2
��4��ѡ���������ֵ�Ч��ġ���Ԫ�ء�X��Y��ԭ���γɳ�������������HCO3����HCO3�� �ͱ���ǿ���ᷴӦ��

��ϰ��ϵ�д�
�����Ŀ
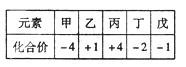
 I�о�G����ż�����壬����˵����ȷ����
I�о�G����ż�����壬����˵����ȷ����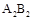
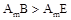
 2BF3(g)������ƽ����ϵ�и����ʵ�Ũ�ȶ����ӵ�ԭ����2����������Ľ����______________________������ţ���
2BF3(g)������ƽ����ϵ�и����ʵ�Ũ�ȶ����ӵ�ԭ����2����������Ľ����______________________������ţ���