��Ŀ����
�������ֶ���������Ԫ��A��B��C�� D��E����ԭ��������������Aԭ��Լռ������ԭ��������88.6%��A+�ֳ�Ϊ���ӣ�B���γɻ�������������Ԫ�أ�CԪ�ص�����⻯��Y��ˮ��Һ�Լ��ԣ�E�Ƕ�����Ԫ���е縺����С��Ԫ�ء�A��B��C��E����Ԫ�ض�����DԪ���γ�ԭ�Ӹ����Ȳ���ͬ�ij���������Իش��������⣺
��1��д��A��E��Ԫ���γɵ�ԭ�Ӹ�����Ϊ1:1�Ļ�����ĵ���ʽ____��
��2�����Ȼ�������Һ�μӹ�����E������������Ӧˮ�������Һ��������____��
��3��Y��Һ�Լ��Ե�ԭ����(��һ�����ӷ���ʽ��ʾ��____��
��4����������β���к��еĻ�����BD�ķ����ǣ�������PdC12��Һ��ͨA����β���������ɺ�ɫ������Pd����֤������β���к���BD��д����Ӧ�����ӷ���ʽ____��
��5�������й��������ʵıȽ��У�����ȷ���� ��
a�����ȶ��ԣ�H2S>SiH4 b�����Ӱ뾶��Na+>S2��
c����һ������N>O d��Ԫ�ص縺�ԣ�C>H
��6����֪����CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ��H=+49.0kJ/mol
��CH3OH(g)+3/2O2(g)=CO2(g)+2H2O(g) ��H=��192��9kJ/mol
����������ʽ��֪��CH3OH��ȼ����____������ڡ��������ڡ���С�ڡ���192.9kJ/mol����֪ˮ��������Ϊ44 kJ/mol�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ____��
��1��д��A��E��Ԫ���γɵ�ԭ�Ӹ�����Ϊ1:1�Ļ�����ĵ���ʽ____��
��2�����Ȼ�������Һ�μӹ�����E������������Ӧˮ�������Һ��������____��
��3��Y��Һ�Լ��Ե�ԭ����(��һ�����ӷ���ʽ��ʾ��____��
��4����������β���к��еĻ�����BD�ķ����ǣ�������PdC12��Һ��ͨA����β���������ɺ�ɫ������Pd����֤������β���к���BD��д����Ӧ�����ӷ���ʽ____��
��5�������й��������ʵıȽ��У�����ȷ���� ��
a�����ȶ��ԣ�H2S>SiH4 b�����Ӱ뾶��Na+>S2��
c����һ������N>O d��Ԫ�ص縺�ԣ�C>H
��6����֪����CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ��H=+49.0kJ/mol
��CH3OH(g)+3/2O2(g)=CO2(g)+2H2O(g) ��H=��192��9kJ/mol
����������ʽ��֪��CH3OH��ȼ����____������ڡ��������ڡ���С�ڡ���192.9kJ/mol����֪ˮ��������Ϊ44 kJ/mol�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ____��
��14�֣�ÿ��2�֣�
��1��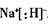
��2�����а�ɫ������Ѹ�ٱ�Ϊ����ɫ������ɺ��ɫ
��3��NH3+H2O NH4++OH-
NH4++OH-
��4��CO+PdCl2+H2O=Pd+CO2+2H++2Cl-
��5��b
��6�����ڣ�H2(g)+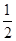 O2(g)=H2O(l) ��H=-124.6KJ/mol
O2(g)=H2O(l) ��H=-124.6KJ/mol
��1��
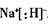
��2�����а�ɫ������Ѹ�ٱ�Ϊ����ɫ������ɺ��ɫ
��3��NH3+H2O
 NH4++OH-
NH4++OH-��4��CO+PdCl2+H2O=Pd+CO2+2H++2Cl-
��5��b
��6�����ڣ�H2(g)+
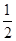 O2(g)=H2O(l) ��H=-124.6KJ/mol
O2(g)=H2O(l) ��H=-124.6KJ/mol����������������������֪A��B��C��D��E�ֱ�ΪH��C��N��O��Na��
��1��Na��H�γ������ͻ�����
��2������������������NaoH��Ϻ����ɰ�ɫ������������������������������
��3��һˮ�ϰ����뷽��ʽ
��4��������ԭ��Ӧ����ʽ��д�����жϷ�Ӧ��������Ȼ����ƽ��
��5��Ԫ�������ɿ�֪���ǽ�����Խǿ����̬�⻯���¶���Խǿ��a��ȷ�����Ӳ���Խ�࣬�뾶Խ��S2->Na+��b����NΪ���������һ�����ܴ���O��c��ȷ���ǽ�����Խǿ���縺��Խ��d��ȷ��
��6���ɢڷ�Ӧ��ˮ����̬��Һ̬���ȿ�֪��CH3OH��ȼ���ȴ���192.9KJ���ɸ�˹���ɿ�֪��������ȼ���ȣ���д���Ȼ�ѧ����ʽ��

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ

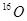 ��
��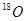 ��Ϊͬλ�أ�
��Ϊͬλ�أ�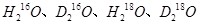 ��Ϊͬ��������
��Ϊͬ��������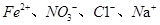 �ܹ���������
�ܹ���������