��Ŀ����
����Ŀ��300mLij��Һ�п��ܺ���Na����NH4����Mg2����Ba2����CO32����SO42����Cl���е������֣��ֽ�����Һ�ֳ����ȷݣ���������ʵ�飺
�����һ���м���AgNO3��Һ���а�ɫ����������
����ڶ����м�����KOH��Һ�����ȣ��ռ�������0.04 mol����������ȿɲ���������
����������м�����BaCl2��Һ���õ�����6.27g���������������ַ�Ӧ��ʣ�����2.33g����������ʵ�飬�ش��������⣺
��1��ʵ��������ɳ��������ӷ���ʽΪ________�������ܽ�����ӷ���ʽΪ__________��
��2����Һ��һ�������ڵ�������_________��
��3��ʵ����м���AgNO3��Һ���г����������ܷ�˵��ԭ��Һ�к���Cl��?________��������������������������______________��
��4���ƶ��������Ƿ���ڲ�˵������_________�������ڣ����������ʵ�����Ũ��____________���������ڣ����ʲ�����
���𰸡�Ba2+ + CO32- === BaCO3����Ba2+ + SO42- === BaSO4�� BaCO3+2H+ === Ba2+ + CO2�� + H2O Mg2+��Ba2+ �� ̼�������������Ȼ�����Ϊ��ɫ��������ȷ���Ƿ���Cl-(��������) ���ڣ����������֪��ֻ�д��������ӣ���Һ�в��ܱ��ֵ����� c(Na+)��0.2mol/L
��������
�����һ���м���AgNO3��Һ���а�ɫ����������˵��CO32-��SO42-��Cl-������һ�֣�����Ba2+��
����ڶ����м�����KOH��Һ�����ȣ��ռ�������0.04 mol��˵����0.04 mol NH4+��
����������м�����BaCl2��Һ���õ�����6.27g���������������ַ�Ӧ��ʣ�����2.33g����SO42-��![]() ����CO32-��
����CO32-��![]() ���ɽ�һ���ж���Mg2+���ٸ��ݵ�����ԭ�ɽ�һ���ж�Na+��Cl-���ڵĿ����ԡ�
���ɽ�һ���ж���Mg2+���ٸ��ݵ�����ԭ�ɽ�һ���ж�Na+��Cl-���ڵĿ����ԡ�
��������ʵ�飬�ش��������⣺
(1)���ݷ�����֪��ʵ��ۼ����Ba2+�ֱ��CO32-��SO42-��Ӧ���ɳ��������ӷ���ʽΪ��Ba2+ + CO32- === BaCO3����Ba2+ + SO42- === Ba SO4����������������BaCO3�ܽ⣬���ӷ���ʽΪ��BaCO3+2H+ === Ba2+ + CO2��+ H2O����Ϊ��Ba2+ + CO32- === BaCO3����Ba2+ + SO42- === BaSO4����BaCO3+2H+ === Ba2+ + CO2�� + H2O
(2)���ݷ�����֪����Һ�к���CO32-��SO42-������Mg2+��Ba2+һ�������ڡ���Ϊ��Mg2+��Ba2+
(3)CO32-��SO42-��Cl- ���ܺ�AgNO3��Һ���ɰ�ɫ����������ʵ����м���AgNO3��Һ���ɰ�ɫ���������ܷ�˵��ԭ��Һ�к���Cl-����Ϊ����̼�������������Ȼ�����Ϊ��ɫ��������ȷ���Ƿ���Cl-(��������)
(4)ÿһ�ȷ��У���֪��������NH4+���������Ϊ0.04 mol����֪��������CO32-��SO42-���������Ϊ0.01 mol ��2+0.02 mol ��2=0.06 mol��������֪������ɣ����ݵ�����ԭ��һ����Na+����Cl-����ȷ��������c(Na+)��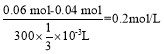 ����Ϊ�����ڣ����������֪��ֻ�д��������ӣ���Һ�в��ܱ��ֵ����ԣ�c(Na+)��0.2mol/L
����Ϊ�����ڣ����������֪��ֻ�д��������ӣ���Һ�в��ܱ��ֵ����ԣ�c(Na+)��0.2mol/L