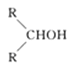
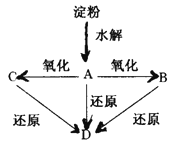
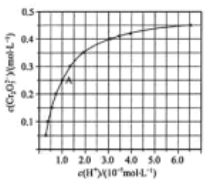
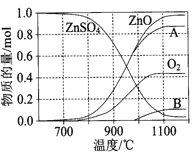
��Ŀ����
����Ŀ���������ʵ�����A��B�������ʻ����2L���ܱ������У��������·�Ӧ��3A��g��+B��g��![]() xC��g��+2D��g������5min���D��Ũ��Ϊ0.5mol/L��c��A����c��B��=3��5��C��ƽ����Ӧ������0.1mol/(L��min)����
xC��g��+2D��g������5min���D��Ũ��Ϊ0.5mol/L��c��A����c��B��=3��5��C��ƽ����Ӧ������0.1mol/(L��min)����
��1����ʱA��Ũ��c��A��=________����Ӧ��ʼǰ����������A��B�����ʵ�����n(A) ��________��n(B) ��________��
��2�� B��ƽ����Ӧ���ʡ�V(B) ��________��
��3�� X��ֵ�Ƕ��٣�X��________
���𰸡�
��1��c(A)=0.75mol��L��1��n(A)=3mol��n(B)=3mol��
��2��v(B)=0.05mol/(L��min)��
��3��x=2��
��������
��������� 3A��g��+B��g��![]() xC��g��+2D��g��
xC��g��+2D��g��
��ʼ�� a a 0 0
�仯�� 1.5 0.5 1x/2 1
ƽ�⣺(a��1.5)(a��0.5) 1x/2 1
����c��A����c��B��=3��5����(a��1.5)��(a��0.5)=3��5�����a=3mol��c(A)=(3��1.5)/2mol��L��1=0.75mol��L��1��v(B)=0.5/(2��5) mol/(L��min)=0.05 mol/(L��min)����������֮�ȵ���ϵ��֮�ȣ�v(B)��v(C)=1��x��0.05��0.1=1��x�����x=2��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�