��Ŀ����
3��Na2S2O3���������Լ�����������ˮ�������ֽ⣮ij�о�С���������ʵ�飺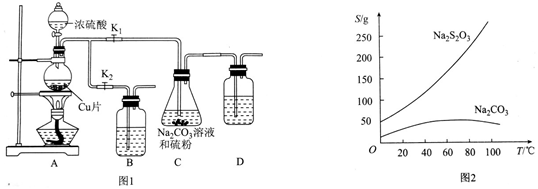
��ʵ��һ��Na2S2O3•5H2O���Ʊ�
I��ʵ��ԭ����Na2SO3��aq��+S��s��$\frac{\underline{\;\;��\;\;}}{\;}$Na2S2O3��aq��
��ʵ��װ�ã���ͼ1��ʾ���й����ʵ��ܽ��������ͼ2��ʾ��
��ʵ�鲽�裺
��1�����װ�������ԣ���ͼ1��ʾ�����Լ�������װ��B��D�мӵ���NaOH��Һ��װ�� C�е����Ӧ������ϸ�����Ҵ���ʪ�������Ӱ�콵�ͷ�Ӧ���ʣ�
��2����K1���ر�K2����Բ����ƿ�м�������Ũ���Ტ���ȣ�C�л��Һ��������������Ӧһ��ʱ�����۵������٣�
��3����C����Һ��pH�ӽ�7ʱ����K2���ر�K1��ֹͣ���ȣ���ȡ�ô�ʩ�������Ƿ�ֹSO2��������Һ�������ԣ��������ɵ�Na2S2O3�ֽ⣮
��4����C�еĻ��Һ���ˣ�����Һ����һϵ�в������ɵôֲ�ƷNa2S2O3•5H2O��
IV����Ʒ��⣺
��5���ֲ�Ʒ�п��ܺ���Na2SO3��Na2SO4�����ʣ����������Լ����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ��ۣ�ȡ������Ʒ��������ϡ���ᣬ���ã�ȡ�ϲ���Һ������ˣ�ȡ��Һ�����μ�BaCl2��Һ�������ֳ�����˵������Na2SO4���ʣ�
��ѡ����Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ
��ʵ���������ˮ�����ȵIJⶨ
����������������ˮ���������Ⱥ����ķ������£���250ml����ƿ���������ƿ���з���0.5gKI����10mlϡ���ᣬȷ��ȡ����ˮ��100ml��������ˮ��ͷ����ˮ����ʮ�����ȡˮ�������ڵ���ƿ��Ѹ��������ҡ������ˮ���ʵ���ɫ����1ml������Һ��������˵��ˮ���������ȣ�����Cmol/L��Na2S2O3��Һ�ζ�������Һ��ɫ��ʧ����ɫ����Һ�����������������Һ�������
����֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪI2+2Na2S2O3�T2NaI+Na2S4O6��
��6����������ˮ����Ư����������˵��ˮ���������ȵķ�Ӧ���ӷ���ʽΪClO-+2I-+2H+=Cl-+I2+H2O��
��7������ʵ�飬���ı�Na2S2O3��ҺV mL��������ˮ��Ʒ����������������Cl2���㣩Ϊ355VCmg•L-1��ʵ���У�����������ҡ�����������������ý��ƫ�ߣ��ƫ�ߡ���ƫ�͡����䡱����
���� ��1��B��D��Ϊ�����ն���Ķ�����������NaOH��Һ���գ�������ϸ������Ӵ�������ӿ췴Ӧ���ʣ�S���ھƾ�������C�е����Ӧ������ϸ�����Ҵ���ʪ������ή�ͷ�Ӧ���ʣ�
��3����ֹSO2��������Һ�������ԣ��������ɵ�Na2S2O3�ֽ⣻
��5��ȡ������Ʒ��������ϡ���ᣬ�������Ȼ�����Һ�����Ƿ�����������ӣ�
��6��Ư�۵���Ч�ɷ��Ǵ�������������������ǿ�����ԣ����Խ������������õⵥ�ʣ���������ԭΪ�����ӣ�
��7������Cl2��I2��2Na2S2O3������������������Ѹ�٣������е����������������¿ɰѵ������������ɵ��ʵ⣬������������Ƶ����������
��� �⣺��1��B��D��Ϊ�����ն���Ķ�����������װ����NaOH��Һ��������ϸ������Ӵ�������ӿ췴Ӧ���ʣ�S���ھƾ�������C�е����Ӧ������ϸ�����Ҵ���ʪ������ή�ͷ�Ӧ���ʣ�
�ʴ�Ϊ��NaOH��Һ�����ͷ�Ӧ���ʣ�
��3��SO2��������Һ�������ԣ��������ɵ�Na2S2O3�ֽ⣬
�ʴ�Ϊ����ֹSO2��������Һ�������ԣ��������ɵ�Na2S2O3�ֽ⣻
��5��������Ʒ���Ƿ��������Ƶķ���Ϊ��ȡ������Ʒ��������ϡ���ᣬ���ã�ȡ�ϲ���Һ������ˣ�ȡ��Һ�����μ�BaCl2��Һ�������ֳ�����˵������Na2SO4���ʣ�
�ʴ�Ϊ��ȡ������Ʒ��������ϡ���ᣬ���ã�ȡ�ϲ���Һ������ˣ�ȡ��Һ�����μ�BaCl2��Һ�������ֳ�����˵������Na2SO4���ʣ�
��6��Ư�۵���Ч�ɷ��Ǵ�������������������ǿ�����ԣ����Խ������������õⵥ�ʣ���������ԭΪ�����ӣ���Ӧ���ӷ���ʽΪ��ClO-+2I-+2H+=Cl-+I2+H2O��
�ʴ�Ϊ��ClO-+2I-+2H+=Cl-+I2+H2O��
��7������Cl2��I2��2Na2S2O3����֪n��Cl2��=$\frac{1}{2}$n��Na2S2O3��=$\frac{1}{2}$��V��10-3L��Cmol/L����������Ϊ=$\frac{1}{2}$��V��10-3L��Cmol/L��71g/mol=3.55VC��10-2g=35.4VC mg������������������Cl2���㣩Ϊ $\frac{35.5VCmg}{0.1L}$=355VC mg/L��
������Ѹ�٣������е����������������¿ɰѵ������������ɵ��ʵ⣬������������Ƶ�������������Խ��ƫ�ߣ�
�ʴ�Ϊ��355VC��ƫ�ߣ�
���� ���⿼�黯ѧʵ����Ʊ��������ⶨ���漰�Բ����ķ������ۡ�β�����ա�����ʽ����д�����ӵļ��顢��ѧ�����֪ʶ���Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

| A�� | ������ѧ��ά���״�����л���ѧ�ĸ���¹���ѧ�ұ�������˹������狀ϳ������أ��״δ������л��������Ľ��� | |
| B�� | ���ϣ��ȼ�շ����ⶨ�л���������̼��Ԫ���������� | |
| C�� | �ú˴Ź������ͺ���������������Ҵ��Ͷ����ѣ�CH3OCH3�� | |
| D�� | �á�ͬλ��ʾ�ٷ������о��л���ѧ��Ӧ���̵��ֶ�֮һ |
| A�� | һ���м��������������м״� | B�� | �������ʶ��� | ||
| C�� | �м��������ͼ��� | D�� | �м״��ͼ��� |
| A�� | 1mol H2Լ����6.02��1023���� | |
| B�� | ˮ��Ħ��������18g | |
| C�� | �Ȼ����Ħ����������������Է������� | |
| D�� | ��ͬ���ʵ�����SO2��SO3������ԭ�Ӹ���֮��Ϊ2��3 |
 ʳ�ξ������������ӣ�ͼ�еġ�
ʳ�ξ������������ӣ�ͼ�еġ� �����������ӣ�ͼ�еġ��𡱣���ɵģ��Ҿ�Ϊ�Ⱦ���Ľ������У���֪ʳ�ε��ܶ���2.2g•cm-3�������ӵ�����6.02��1023 mol-1����ʳ�ξ���������������������������ļ�ľ�����ӽ��ڣ�������
�����������ӣ�ͼ�еġ��𡱣���ɵģ��Ҿ�Ϊ�Ⱦ���Ľ������У���֪ʳ�ε��ܶ���2.2g•cm-3�������ӵ�����6.02��1023 mol-1����ʳ�ξ���������������������������ļ�ľ�����ӽ��ڣ�������| A�� | 3.0��10-8 cm | B�� | 3.5��10-8 cm | C�� | 4.0��10-8 cm | D�� | 5.0��10-8 cm |
| A�� | ��4c-b+2c��kJ | B�� | $\frac{4c-b-2a}{2}$ kJ | C�� | 4c+b-2a kJ | D�� | $\frac{4c+b-2a}{2}$ kJ |
| A�� | 1 mol Fe������������Ӧʱת�Ƶĵ�����Ϊ2NA | |
| B�� | 0.2 mol S�ڿ����г��ȼ�գ�ת�Ƶ�����Ϊ0.6NA | |
| C�� | 0.1molCl2����ˮ�з�����Ӧ��ת�Ƶ�����Ϊ0.1NA | |
| D�� | 1.5 mol Na2O2�������Ķ�����̼��ַ�Ӧ��ת�Ƶ�����Ϊ1.5NA |
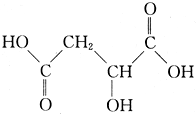 ƻ������һ�ֳ������л��ᣬ��ṹ��ʽ��ͼ��ʾ��
ƻ������һ�ֳ������л��ᣬ��ṹ��ʽ��ͼ��ʾ��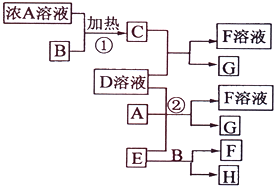 �й����ʴ�����ͼ��ʾ��ת����ϵ�����ֲ�����ʡ�ԣ���ͨ��CΪ���嵥�ʣ�GΪ�Ϻ�ɫ���嵥�ʣ�ʵ�����У����ù���E��B�Ĵ��¼�����ȡ���嵥��H��
�й����ʴ�����ͼ��ʾ��ת����ϵ�����ֲ�����ʡ�ԣ���ͨ��CΪ���嵥�ʣ�GΪ�Ϻ�ɫ���嵥�ʣ�ʵ�����У����ù���E��B�Ĵ��¼�����ȡ���嵥��H��